Continuous-Flow Electron Spin Resonance Measurements of Hydroxyl Radicals Produced during Photocatalytic Water Oxidation
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Hydroxyl radicals (•OH) generated on semiconductor oxide photocatalysts are expected to facilitate the decomposition and selective oxidation of organic compounds. However, the efficiency and behavior of photocatalytic •OH production have not been fully understood. In this study, we developed a flow system in which an electrolyte containing the spin-trapping agent, 5,5-dimethyl-1-pyrroline-N-oxide (DMPO), was passed through a photoelectrochemical cell, and the radical species generated by the photoanodic water oxidation were continuously analyzed by electron spin resonance (ESR) spectroscopy. This flow-based ESR measurement enabled us to simultaneously quantify the current density and radical production rate under photocatalytic reaction, and to measure the Faradaic efficiency (FE) for the formation of the spin adduct (•DMPO-OH) with near-real-time response. We utilized a tungsten oxide (WO3) electrode as a typical photocatalyst to investigate •OH formation during water oxidation. When the incident photon-to-photocurrent conversion efficiency (IPCE) was 7.1 to 12.7% at an electrode potential of 1.20 V vs RHE, the FE of •OH formation was found to be low, ranging from 0.63 to 0.92%. The •OH FE increased with decreasing applied electrode potential or light intensity, suggesting that the surface density of photogenerated holes may influence the •OH formation.
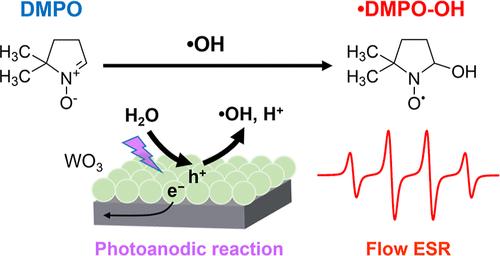
连续流电子自旋共振测量光催化水氧化过程中产生的羟基自由基
在半导体氧化物光催化剂上产生的羟基自由基(-OH)有望促进有机化合物的分解和选择性氧化。然而,人们对光催化产生 -OH 的效率和行为尚未完全了解。在这项研究中,我们开发了一种流动系统,其中含有自旋捕获剂 5,5-二甲基-1-吡咯啉-N-氧化物(DMPO)的电解质通过光电化学电池,通过电子自旋共振(ESR)光谱连续分析光阳极水氧化产生的自由基物种。这种基于流动的 ESR 测量使我们能够同时量化光催化反应中的电流密度和自由基产生率,并以近乎实时的响应测量形成自旋加合物(-DMPO-OH)的法拉第效率(FE)。我们利用氧化钨(WO3)电极作为典型的光催化剂来研究水氧化过程中 -OH 的形成。当电极电位为 1.20 V vs RHE 时,入射光子-光电流转换效率(IPCE)为 7.1% 至 12.7%,发现 -OH 形成的 FE 很低,为 0.63% 至 0.92%。随着应用电极电位或光照强度的降低,-OH 的 FE 增加,这表明光生孔的表面密度可能会影响-OH 的形成。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


