Enhancing Catalytic Performance in Oxidative Steam Reforming of Ethanol: The Role of Ruthenium ion Substitution in Layered Perovskite La2Ti2–xRuxO7±δ Catalysts
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
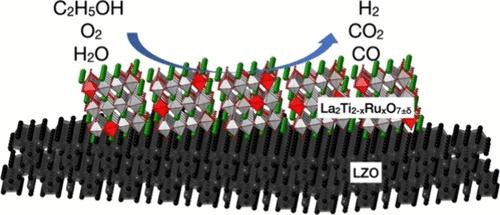
Layered perovskite oxides of La2Ti2–xRuxO7±δ, where x ranges from 0.1 to 0.4 (LTR01–04), demonstrate significant catalytic efficiency and stability in the oxidative steam reforming of ethanol (OSRE). The LTR03 catalyst (0.50 wt % Ru) achieves optimal performance, achieving complete ethanol conversion and an exceptional hydrogen selectivity rate of 100.0(5)% under conditions of C/O = 0.6, GHSV = 120,000 h–1, and a furnace temperature of 400 °C. When paired with La2Zr2O7 as a support material, the LTR03 catalyst maintains a stable hydrogen selectivity of 98(1)% and an ethanol conversion rate of 99(1)% at a C/O ratio of 0.6, without any noticeable carbon deposition, over a 120 h OSRE activity test. Investigations using X-ray photoelectron Spectra (XPS), X-ray absorption spectroscopy (XAS), temperature-programmed reduction (TPR), synchrotron, and neutron powder diffraction indicate that the partial substitution of ruthenium cations leads to the emergence of multivalence of Run+/Ru4+ ions and triggers the creation of oxygen vacancies and boosting the OSRE performance of La2Ti2–xRuxO7±δ. Rietveld analyses of powder diffraction data disclose a site-specific preference for Run+ and Ti4+ ions within the metal lattice. In-situ powder X-ray diffraction (PXRD) and neutron powder diffraction studies on both the regular and reduced forms of La2Ti1.7Ru0.3O7±δ (LTR03) reveal that oxygen vacancies predominantly form at the top and bottom regions of the [M2O7]n− layer. These vacancies are crucial for effectively converting ethanol and hydrocarbons in the OSRE process. These findings pave the way for further research into metal-substituted layered perovskites as catalysts and La2Zr2O7 as a supporting material for efficient hydrogen production via ethanol conversion in the OSRE process.
提高乙醇氧化蒸汽转化的催化性能:层状过氧化物 La2Ti2-xRuxO7±δ 催化剂中钌离子置换的作用
La2Ti2-xRuxO7±δ层状包晶氧化物(其中 x 在 0.1 至 0.4 之间)(LTR01-04)在乙醇氧化蒸汽转化(OSRE)中表现出显著的催化效率和稳定性。LTR03 催化剂(0.50 wt % Ru)实现了最佳性能,在 C/O = 0.6、GHSV = 120,000 h-1 和炉温 400 °C 的条件下,实现了乙醇的完全转化和高达 100.0(5)% 的优异氢气选择性。与 La2Zr2O7 作为支撑材料搭配使用时,LTR03 催化剂在 C/O 比为 0.6 的条件下保持稳定的氢气选择率 98(1)%,乙醇转化率 99(1)%,并且在 120 小时的 OSRE 活性测试中没有任何明显的碳沉积。利用 X 射线光电子能谱 (XPS)、X 射线吸收光谱 (XAS)、温度编程还原 (TPR)、同步辐射和中子粉末衍射进行的研究表明,钌阳离子的部分取代导致 Run+/Ru4+ 离子的多价出现,并引发氧空位的产生,从而提高了 La2Ti2-xRuxO7±δ 的 OSRE 性能。对粉末衍射数据进行的里特维尔德分析表明,金属晶格内的 Run+ 和 Ti4+ 离子具有特定的偏好位点。对正态和还原态 La2Ti1.7Ru0.3O7±δ (LTR03) 进行的原位粉末 X 射线衍射 (PXRD) 和中子粉末衍射研究表明,氧空位主要形成于 [M2O7]n- 层的顶部和底部区域。这些空位对于在 OSRE 过程中有效转化乙醇和碳氢化合物至关重要。这些发现为进一步研究作为催化剂的金属取代层状过氧化物和作为支撑材料的 La2Zr2O7 在 OSRE 过程中通过乙醇转化高效制氢铺平了道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


