Ad-Hoc Functionalization of Phenothiazine Dioxide Derivatives to Achieve Blue Thermally Activated Delayed Fluorescence in Organic Nanoaggregates
IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
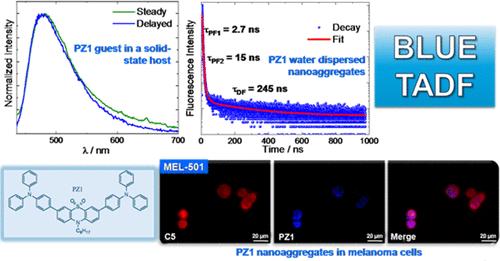
In this study, four new phenothiazine dioxide derivatives were synthesized. They were functionalized with rotatable electron donor substituents characterized by different degree of rotatability (such as triphenylamine, phenothiazine and tetraphenylethylene) symmetrically attached to the central core. On the one hand, the triphenylamine- and phenothiazine-based molecules exhibited efficient fluorescence in the blue portion of the visible spectrum, consistent with their modest push–pull character. On the other hand, the tetraphenylethylene-functionalized compound showed very low fluorescence quantum yields and an ultrafast nonradiative deactivation to the ground state via intramolecular rotations. Efficient intersystem crossing (ISC) was revealed through transient absorption experiments with femtosecond and nanosecond temporal resolution for the triphenylamine and phenothiazine derivatives. Organic nanoaggregates of the investigated fluorophores with mean diameters of 50–150 nm were prepared in water dispersion and intriguingly exhibited either blue thermally activated delayed fluorescence (TADF, for the triphenylamine-based dye) or aggregation-induced emission (AIE, for the tetraphenylethylene-based dye). Such organic nanoparticles were successfully internalized in human melanoma and lung cancer cells, exhibiting nuclear localization via fluorescence imaging and some cytotoxicity. TADF was interestingly revealed for the triphenylamine-functionalized molecule also in the solid state, in host–guest powders where triphenylphosphine was employed as the host matrix. This new blue TADF emitter holds significant potential for applications in third- and fourth-generation OLED devices.
对二氧化吩噻嗪衍生物进行临时功能化,在有机纳米聚集体中实现蓝色热激活延迟荧光
本研究合成了四种新的二氧化吩噻嗪衍生物。这些衍生物的中心核对称地连接了可旋转电子供体取代基(如三苯胺、吩噻嗪和四苯基乙烯)。一方面,基于三苯胺和吩噻嗪的分子在可见光谱的蓝色部分显示出高效荧光,这与其适度的推拉特性相一致。另一方面,四苯乙烯功能化化合物的荧光量子产率非常低,并通过分子内旋转超快地非辐射去活化到基态。通过对三苯胺和吩噻嗪衍生物进行飞秒和纳秒时间分辨率的瞬态吸收实验,发现了高效的系统间交叉(ISC)。在水分散液中制备了平均直径为 50-150 纳米的所研究荧光团的有机纳米聚集体,这些聚集体令人感兴趣地显示出蓝色热激活延迟荧光(TADF,三苯胺基染料)或聚集诱导发射(AIE,四苯乙烯基染料)。这种有机纳米粒子成功地在人类黑色素瘤和肺癌细胞中被内化,通过荧光成像显示出核定位和一定的细胞毒性。有趣的是,三苯胺功能化分子还在固态下,在以三苯基膦为基质的主客体粉末中显示出 TADF。这种新型蓝色 TADF 发射器在第三代和第四代有机发光二极管设备中的应用潜力巨大。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


