The Physical Driving Forces of Conformational Transition for TTR91–96 with Proline Mutations
IF 5.3
2区 化学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Pathological aggregation of essentially dissociated Transthyretin (TTR) monomer proteins, driven by misfolding and self-interaction, is associated with Transthyretin amyloidosis (ATTR) disease. The TTR monomer proteins consist of several fragments that tend to self-aggregate. Recent experimental studies showed that the sequence of residues TTR91–96 plays an important role in self-aggregation. However, the mechanisms underlying the misfolding and aggregation of the TTR91–96 monomers are still unknown. In this study, we used microsecond molecular dynamics simulations to investigate the misfolding and self-assembly of TTR91–96 Octamers. We also investigated E92P and V94P mutants for comparative analysis. The analysis indicates that hydrophobic interactions and π–π stacking patterns play important roles in reducing the β-sheet content in the V94P and E92P mutants. Additionally, our findings reveal the conformational transition of TTR91–96 octamer from closed β-barrel, open β-barrel to the β-bilayer aggregation. We further elucidate the dynamic mechanism of the transition from intermediate states to stable states. Overall, our research may contribute to the development of drug design to combat fibrous amyloid fibrous diseases.
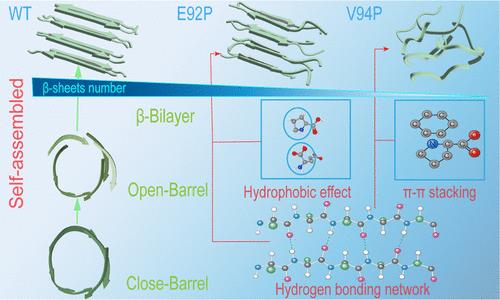
脯氨酸突变的 TTR91-96 构象转变的物理驱动力
在错误折叠和自我交互作用的驱动下,基本解离的Transthyretin(TTR)单体蛋白发生病理性聚集,这与Transthyretin淀粉样变性(ATTR)疾病有关。TTR 单体蛋白由多个片段组成,这些片段往往会自我聚集。最近的实验研究表明,残基 TTR91-96 的序列在自聚集中起着重要作用。然而,TTR91-96 单体错误折叠和聚集的机制仍然未知。在本研究中,我们利用微秒分子动力学模拟研究了 TTR91-96 八聚体的错误折叠和自组装。我们还对 E92P 和 V94P 突变体进行了比较分析。分析表明,疏水相互作用和π-π堆叠模式在减少 V94P 和 E92P 突变体中的β片层含量方面发挥了重要作用。此外,我们的研究结果揭示了 TTR91-96 八聚体从封闭的 β 管、开放的 β 管到 β 层聚集的构象转变。我们进一步阐明了从中间态过渡到稳定态的动态机制。总之,我们的研究可能有助于开发防治淀粉样纤维疾病的药物设计。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.80
自引率
10.70%
发文量
529
审稿时长
1.4 months
期刊介绍:
The Journal of Chemical Information and Modeling publishes papers reporting new methodology and/or important applications in the fields of chemical informatics and molecular modeling. Specific topics include the representation and computer-based searching of chemical databases, molecular modeling, computer-aided molecular design of new materials, catalysts, or ligands, development of new computational methods or efficient algorithms for chemical software, and biopharmaceutical chemistry including analyses of biological activity and other issues related to drug discovery.
Astute chemists, computer scientists, and information specialists look to this monthly’s insightful research studies, programming innovations, and software reviews to keep current with advances in this integral, multidisciplinary field.
As a subscriber you’ll stay abreast of database search systems, use of graph theory in chemical problems, substructure search systems, pattern recognition and clustering, analysis of chemical and physical data, molecular modeling, graphics and natural language interfaces, bibliometric and citation analysis, and synthesis design and reactions databases.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


