Incretin-based therapies for the treatment of obesity-related diseases
引用次数: 0
Abstract
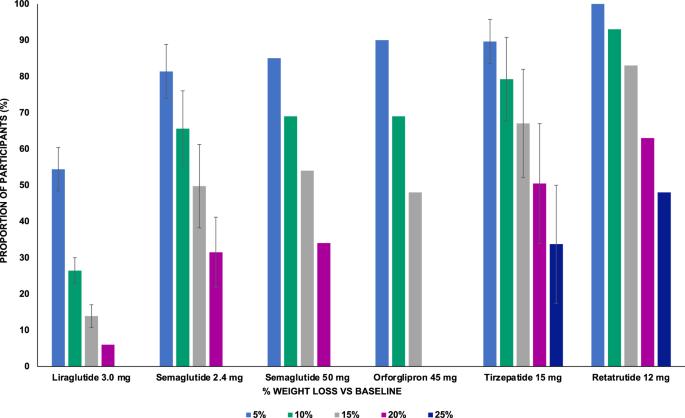
Obesity-related disability-adjusted life years (DALYs) are expected to increase by approximately 40% from 2020 to 2030. DALYs and mortality related to obesity are the consequence of multiple comorbidities such as cardiovascular (i.e., heart failure) and metabolic diseases (i.e. type 2 diabetes [T2D], metabolic dysfunction-associated steatotic liver disease [MASLD]). Lifestyle interventions represent the foundation of obesity treatment, yet an escalation to pharmacological and/or surgical interventions is often needed. Liraglutide, semaglutide and tirzepatide are incretin-based therapies currently approved by FDA for the management of obesity, while triple GIPR/GCGR/GLP-1R agonist retatrutide (LY3437943), the cagrilintide/semaglutide (CagriSema) 2.4 mg combination, high-dose oral semaglutide, and oral orforglipron are in advanced stages of development. Incretin-based therapies have been associated with a body weight (BW) reduction of ≥5% in at least half of patients in most randomized controlled trials (RCT) and real-world studies (RWS). Semaglutide and tirzepatide have also displayed a mean 60–69% 10-years relative risk reduction of T2D development. In line with evidence accrued in patients with T2D, incretin-based therapies produced a favorable effect on traditional cardiovascular risk factors, such as lipids and blood pressure, and even reduced the risk of major cardiovascular events and heart failure-related events in individuals with obesity, as recently demonstrated for the first time in the SELECT trial with semaglutide 2.4 mg once-weekly. Moreover, incretin-based therapies have also been proven beneficial on obesity-related comorbidities, such as knee osteoarthritis (KOA), obstructive sleep apnea (OSA) syndrome, and MASLD. Further research is needed to improve our understanding of their effects on obesity-related comorbidities and the underlying mechanism, whether involving direct effects on target tissues or mediated by improvement in BW, glucose levels and other CV risk factors.
治疗肥胖相关疾病的内分泌疗法
预计从 2020 年到 2030 年,与肥胖相关的残疾调整生命年(DALYs)将增加约 40%。与肥胖相关的残疾调整寿命年数和死亡率是多种并发症的结果,如心血管疾病(即心力衰竭)和代谢性疾病(即 2 型糖尿病、代谢功能障碍相关性脂肪肝)。生活方式干预是肥胖症治疗的基础,但往往需要升级到药物和/或手术干预。利拉鲁肽、司马鲁肽和替泽帕肽是目前美国食品及药物管理局批准用于治疗肥胖症的增量素疗法,而三重 GIPR/GCGR/GLP-1R 激动剂雷塔鲁肽(LY3437943)、卡格列林肽/司马鲁肽(CagriSema)2.4 毫克复方制剂、大剂量口服司马鲁肽和口服奥弗利普隆则处于后期开发阶段。在大多数随机对照试验(RCT)和真实世界研究(RWS)中,基于胰岛素的疗法可使至少一半患者的体重(BW)下降≥5%。塞马鲁肽和替唑帕肽还显示,T2D发病的10年相对风险平均降低了60-69%。与在 T2D 患者中积累的证据一致,基于增量胰岛素的疗法对血脂和血压等传统心血管风险因素产生了有利影响,甚至降低了肥胖症患者发生重大心血管事件和心衰相关事件的风险,最近在 SELECT 试验中首次使用了 2.4 毫克、每周一次的塞马鲁肽。此外,基于增量素的疗法还被证明对肥胖相关的合并症有益,如膝关节骨关节炎(KOA)、阻塞性睡眠呼吸暂停(OSA)综合征和MASLD。我们需要开展进一步的研究,以更好地了解它们对肥胖相关合并症的影响及其内在机制,无论是对靶组织的直接影响,还是通过改善体重、血糖水平和其他心血管疾病风险因素而介导的影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


