Localized release of muscle-generated BDNF regulates the initial formation of postsynaptic apparatus at neuromuscular synapses
IF 15.4
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
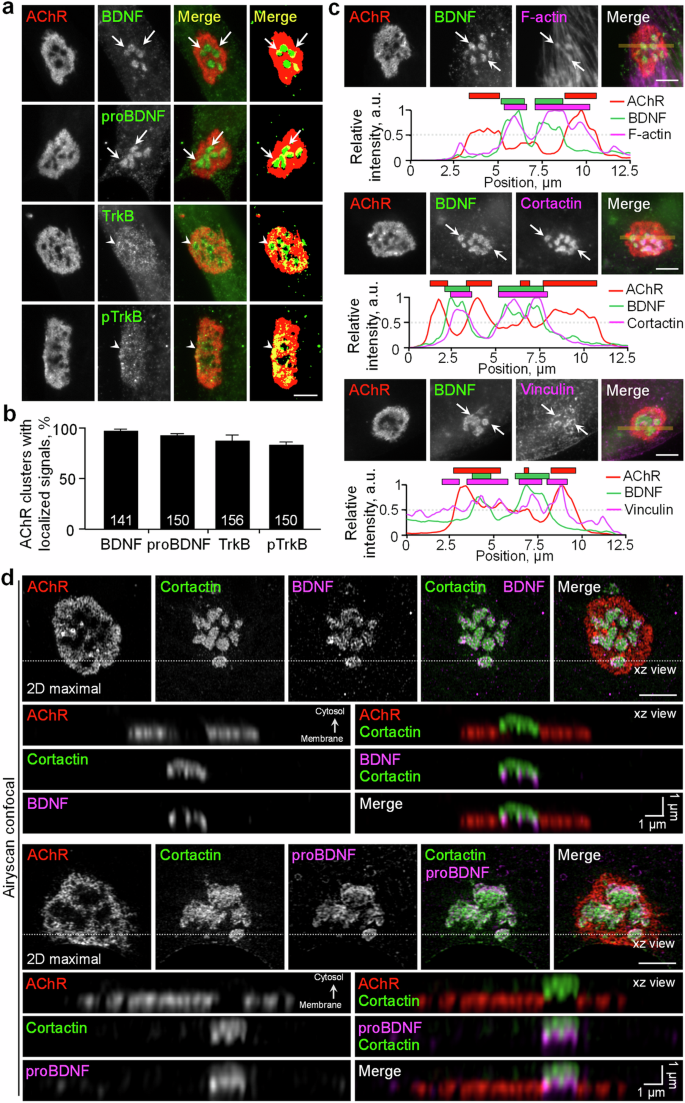
Growing evidence indicates that brain-derived neurotrophic factor (BDNF) is produced in contracting skeletal muscles and is secreted as a myokine that plays an important role in muscle metabolism. However, the involvement of muscle-generated BDNF and the regulation of its vesicular trafficking, localization, proteolytic processing, and spatially restricted release during the development of vertebrate neuromuscular junctions (NMJs) remain largely unknown. In this study, we first reported that BDNF is spatially associated with the actin-rich core domain of podosome-like structures (PLSs) at topologically complex acetylcholine receptor (AChR) clusters in cultured Xenopus muscle cells. The release of spatially localized BDNF is tightly controlled by activity-regulated mechanisms in a calcium-dependent manner. Live-cell time-lapse imaging further showed that BDNF-containing vesicles are transported to and captured at PLSs in both aneural and synaptic AChR clusters for spatially restricted release. Functionally, BDNF knockdown or furin-mediated endoproteolytic activity inhibition significantly suppresses aneural AChR cluster formation, which in turn affects synaptic AChR clustering induced by nerve innervation or agrin-coated beads. Lastly, skeletal muscle-specific BDNF knockout (MBKO) mice exhibit structural defects in the formation of aneural AChR clusters and their subsequent recruitment to nerve-induced synaptic AChR clusters during the initial stages of NMJ development in vivo. Together, this study demonstrated the regulatory roles of PLSs in the intracellular trafficking, spatial localization, and activity-dependent release of BDNF in muscle cells and revealed the involvement of muscle-generated BDNF and its proteolytic conversion in regulating the initial formation of aneural and synaptic AChR clusters during early NMJ development in vitro and in vivo.

肌肉产生的 BDNF 的局部释放调节神经肌肉突触后装置的初步形成
越来越多的证据表明,脑源性神经营养因子(BDNF)在收缩的骨骼肌中产生,并作为肌动素分泌,在肌肉代谢中发挥重要作用。然而,在脊椎动物神经肌肉接头(NMJs)的发育过程中,肌肉产生的 BDNF 的参与及其囊泡运输、定位、蛋白水解处理和空间限制释放的调控在很大程度上仍然未知。在这项研究中,我们首次报道了 BDNF 在培养的爪蟾肌肉细胞中与拓扑结构复杂的乙酰胆碱受体(AChR)簇的荚膜样结构(PLSs)富含肌动蛋白的核心结构域存在空间关联。空间定位的 BDNF 的释放受钙依赖性活动调节机制的严格控制。活细胞延时成像进一步表明,含有BDNF的囊泡被运输到神经节和突触AChR簇的PLS并被捕获,以进行空间限制性释放。从功能上讲,敲除 BDNF 或抑制 furin 介导的内切蛋白水解活性可显著抑制神经节 AChR 簇的形成,进而影响神经支配或琼脂糖包被珠诱导的突触 AChR 簇。最后,骨骼肌特异性 BDNF 基因敲除(MBKO)小鼠在体内 NMJ 发育的初始阶段表现出神经节 AChR 簇的形成及其随后被招募到神经诱导的突触 AChR 簇的结构缺陷。总之,这项研究证明了 PLSs 在肌肉细胞内 BDNF 的胞内转运、空间定位和活动依赖性释放中的调控作用,并揭示了肌肉生成的 BDNF 及其蛋白水解转换参与了体外和体内 NMJ 早期发育过程中神经节和突触 AChR 团簇初始形成的调控。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Death and Differentiation
生物-生化与分子生物学
CiteScore
24.70
自引率
1.60%
发文量
181
审稿时长
3 months
期刊介绍:
Mission, vision and values of Cell Death & Differentiation:
To devote itself to scientific excellence in the field of cell biology, molecular biology, and biochemistry of cell death and disease.
To provide a unified forum for scientists and clinical researchers
It is committed to the rapid publication of high quality original papers relating to these subjects, together with topical, usually solicited, reviews, meeting reports, editorial correspondence and occasional commentaries on controversial and scientifically informative issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


