Itaconate transporter SLC13A3 impairs tumor immunity via endowing ferroptosis resistance
IF 48.8
1区 医学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
Immune checkpoint blockade (ICB) triggers tumor ferroptosis. However, most patients are unresponsive to ICB. Tumors might evade ferroptosis in the tumor microenvironment (TME). Here, we discover SLC13A3 is an itaconate transporter in tumor cells and endows tumor ferroptosis resistance, diminishing tumor immunity and ICB efficacy. Mechanistically, tumor cells uptake itaconate via SLC13A3 from tumor-associated macrophages (TAMs), thereby activating the NRF2-SLC7A11 pathway and escaping from immune-mediated ferroptosis. Structural modeling and molecular docking analysis identify a functional inhibitor for SLC13A3 (SLC13A3i). Deletion of ACOD1 (an essential enzyme for itaconate synthesis) in macrophages, genetic ablation of SLC13A3 in tumors, or treatment with SLC13A3i sensitize tumors to ferroptosis, curb tumor progression, and bolster ICB effectiveness. Thus, we identify the interplay between tumors and TAMs via the SLC13A3-itaconate-NRF2-SLC7A11 axis as a previously unknown immune ferroptosis resistant mechanism in the TME and SLC13A3 as a promising immunometabolic target for treating SLC13A3+ cancer.
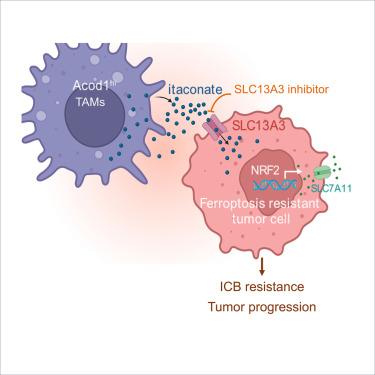
衣康酸转运体 SLC13A3 通过赋予铁变态反应抗性损害肿瘤免疫力
免疫检查点阻断疗法(ICB)可引发肿瘤铁质化。然而,大多数患者对 ICB 没有反应。肿瘤可能会在肿瘤微环境(TME)中逃避铁凋亡。在这里,我们发现 SLC13A3 是肿瘤细胞中的一种伊他康酸转运体,并赋予肿瘤抗嗜铁性,降低肿瘤免疫力和 ICB 的疗效。从机理上讲,肿瘤细胞通过 SLC13A3 从肿瘤相关巨噬细胞(TAMs)中吸收伊他康酸,从而激活 NRF2-SLC7A11 通路,摆脱免疫介导的铁中毒。结构建模和分子对接分析确定了一种 SLC13A3 的功能性抑制剂(SLC13A3i)。在巨噬细胞中缺失 ACOD1(伊他康酸合成所必需的酶)、对肿瘤中的 SLC13A3 进行基因消减或用 SLC13A3i 治疗,都会使肿瘤对铁蛋白沉积敏感、抑制肿瘤进展并增强 ICB 的有效性。因此,我们发现肿瘤和TAMs之间通过SLC13A3-itaconate-NRF2-SLC7A11轴的相互作用是先前未知的TME中免疫铁凋亡抗性机制,而SLC13A3是治疗SLC13A3+癌症的一个有希望的免疫代谢靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer Cell
医学-肿瘤学
CiteScore
55.20
自引率
1.20%
发文量
179
审稿时长
4-8 weeks
期刊介绍:
Cancer Cell is a journal that focuses on promoting major advances in cancer research and oncology. The primary criteria for considering manuscripts are as follows:
Major advances: Manuscripts should provide significant advancements in answering important questions related to naturally occurring cancers.
Translational research: The journal welcomes translational research, which involves the application of basic scientific findings to human health and clinical practice.
Clinical investigations: Cancer Cell is interested in publishing clinical investigations that contribute to establishing new paradigms in the treatment, diagnosis, or prevention of cancers.
Insights into cancer biology: The journal values clinical investigations that provide important insights into cancer biology beyond what has been revealed by preclinical studies.
Mechanism-based proof-of-principle studies: Cancer Cell encourages the publication of mechanism-based proof-of-principle clinical studies, which demonstrate the feasibility of a specific therapeutic approach or diagnostic test.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


