Reductive Coupling of N-Heteroarenes and 1,2-Dicarbonyls for Direct Access to γ-Amino Acids, Esters, and Ketones Using a Heterogeneous Single-Atom Iridium Catalyst
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Despite their significant importance, the challenges in direct and diverse synthesis of N-heterocyclic γ-amino acids/esters/ketones hamper exploration of their applications. Herein, by developing a multifunctional heterogeneous iridium single-atom catalyst composed of silica-confined iridium species and a boron-doped ZrO2 support (Ir-SAs@B-ZrO2/SiO2), we describe its utility in establishing a new reductive coupling reaction of N-heteroarenes and 1,2-dicarbonyls for selective and diverse construction of the as-described compounds in a straightforward manner. The striking features, including good substrate and functionality tolerance, high step and atom economy, exceptional catalyst reusability, and diversified product post-transformations, highlight the practicality of the developed chemistry. Mechanistic studies reveal that the synergy between the active Ir sites and acidic support favors a chemoselective reduction of the more inert N-heteroarenes and affords requisite enamine intermediates. In this work, the concept on precise transformation of reductive intermediates will open a door to further develop useful tandem reactions by rational catalyst design.
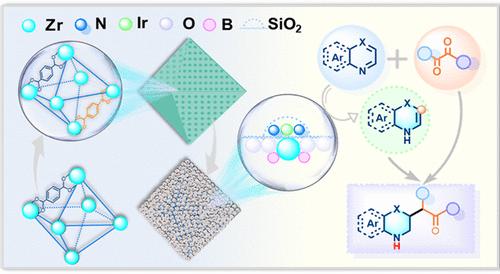
使用异构单原子铱催化剂还原偶联 N-杂环烯和 1,2-二羰基以直接获得 γ-氨基酸、酯和酮
尽管N-杂环γ-氨基酸/酯/酮具有重要意义,但其直接和多样化合成过程中存在的挑战阻碍了对其应用的探索。在本文中,我们通过开发一种由二氧化硅封闭铱物种和掺硼 ZrO2 载体(Ir-SAs@B-ZrO2/SiO2)组成的多功能异质铱单原子催化剂,描述了它在建立 N-heteroarenes 和 1,2-二羰基的新型还原偶联反应中的作用,从而以直接的方式选择性地、多样化地构建所述化合物。该反应具有显著的特点,包括对底物和官能度的良好耐受性、高步长和原子经济性、催化剂的卓越可重复使用性以及多样化的产物后转化,突出了所开发化学反应的实用性。机理研究表明,活性铱位点和酸性载体之间的协同作用有利于惰性 N- 异戊二烯的化学选择性还原,并产生必要的烯胺中间体。在这项工作中,精确转化还原性中间体的概念将为通过合理的催化剂设计进一步开发有用的串联反应打开一扇大门。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


