Thermal Degradation Mechanism and Reaction Kinetics of CsPbBr3 Nanocrystals Using the Coats–Redfern Method
IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
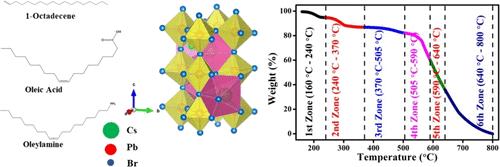
Cesium lead halide perovskite nanocrystals (NCs) are highly regarded for their potential in solar cells, photodetectors, and X/γ-ray detectors. However, their moisture sensitivity and thermal instability limit their applications, which entail a thorough understanding of their thermal degradation mechanisms. In this study, we investigate the thermal degradation kinetics of CsPbBr3 NCs spanning temperatures from 25 to 800 °C using thermogravimetric analysis (TGA). The Coats–Redfern method was employed to analyze the kinetics across various models, determining key parameters such as the correlation coefficient (R2), activation energy (Ea), and pre-exponential factor (A). Differential thermogravimetric (DTG) analysis revealed six distinct peaks, indicating six major thermal events. Consequently, the weight loss curve was divided into six zones, and kinetic parameters were calculated for each zone using multiple kinetic models. Utilizing the kinetic triplet, we further determined the thermodynamic parameters including enthalpy change (ΔH), entropy change (ΔS), and Gibbs free energy change (ΔG) for each zone. This comprehensive analysis enhances the understanding of the thermal stability and degradation mechanisms of CsPbBr3, paving the way for advanced applications in various fields.
使用 Coats-Redfern 法研究硒硼烷纳米晶体的热降解机理和反应动力学
卤化铯铅过氧化物纳米晶体(NCs)因其在太阳能电池、光电探测器和 X/γ 射线探测器中的应用潜力而备受推崇。然而,它们对湿气的敏感性和热不稳定性限制了它们的应用,这就需要对它们的热降解机制有透彻的了解。在本研究中,我们使用热重分析法(TGA)研究了 CsPbBr3 NCs 在 25 至 800 °C 温度范围内的热降解动力学。我们采用 Coats-Redfern 法分析了各种模型的动力学,确定了相关系数 (R2)、活化能 (Ea) 和预指数因子 (A) 等关键参数。差示热重(DTG)分析显示了六个不同的峰值,表明了六个主要的热事件。因此,失重曲线被划分为六个区域,并使用多种动力学模型计算了每个区域的动力学参数。利用动力学三元组,我们进一步确定了每个区域的热力学参数,包括焓变 (ΔH)、熵变 (ΔS)和吉布斯自由能变化 (ΔG)。这项全面的分析加深了人们对 CsPbBr3 热稳定性和降解机制的理解,为其在各个领域的先进应用铺平了道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


