Insights into the Reactivity of the Mn3O4(001) Thin Film with Water
IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
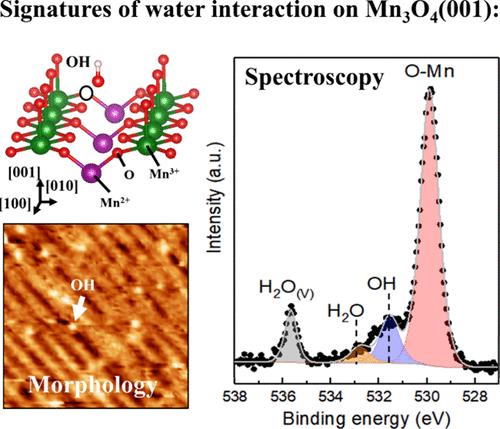
The correlation between the geometric structure and reactivity of an oxide surface is crucial to designing new catalytic materials. This work uses a well-ordered Mn3O4(001) thin film phase to investigate water-oxide interaction. Based on scanning tunneling microscopy data, we propose that water molecule adsorption occurs at oxygen-defective sites and is followed by water dissociation. The vibrational modes related to the D2O (heavy water) physisorbed and the chemisorbed OsD on the surface were identified by infrared reflection absorption spectroscopy at the cryogenic temperature range. Photoelectron spectroscopy at near ambient pressure shows that the water adsorption at defective sites reaches a saturation condition. For water pressures lower than 1 × 10–4 mbar, the crystalline structure of the Mn3O4(001) thin film is preserved. Temperature-programmed spectroscopy probed the desorption process and showed that the water interaction induces a phase transition from Mn3O4 to MnO.
透视 Mn3O4(001) 薄膜与水的反应性
氧化物表面的几何结构与反应活性之间的相关性对于设计新型催化材料至关重要。这项研究利用有序的 Mn3O4(001)薄膜相来研究水与氧化物的相互作用。根据扫描隧道显微镜数据,我们提出水分子吸附发生在氧缺陷位点,随后水解离。在低温范围内,通过红外反射吸收光谱确定了与表面上物理吸附的 D2O(重水)和化学吸附的 OsD 有关的振动模式。近环境压力下的光电子能谱显示,缺陷位点的水吸附达到了饱和状态。当水压低于 1 × 10-4 毫巴时,Mn3O4(001) 薄膜的结晶结构保持不变。温度编程光谱探测了解吸过程,结果表明水的相互作用引起了从 Mn3O4 到 MnO 的相变。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


