Theoretical Investigation of the Steric Effects on Alkyne Semihydrogenation Catalyzed by Frustrated Lewis Pairs
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Frustrated Lewis pairs (FLPs) have received increasing attention for offering a distinctive pathway for the activation and conversion of small molecules. Here, we employ density functional calculations to investigate the electronic and steric effects of FLPs and alkynes on the activity toward alkyne semihydrogenation, a crucial reaction in the production of pharmaceuticals and polymers. We investigated the steric effect of FLPs by replacing the LA-LB linker (d) of the model catalyst FLP, 1-NMe2-2-B(C6F5)2-C6H4 (d1), with various linkers. Additionally, we studied the steric effect of alkynes by varying the substrate from acetylene to but-2-yne, hex-3-yne, 2,5-dimethylhex-3-yne, 2,2,5,5-tetramethylhex-3-yne, and 1,2-diphenylethyne. Our computational results suggest that the overall activity of alkyne and alkene hydrogenation is influenced by both electronic and steric effects when using FLPs with varied LA-LB linkers. To achieve better activity, one could increase the steric hindrance of the LA neighboring environment and reduce the electron density of the LB site of FLPs. Structure–activity relationships for each elementary step involving alkyne semihydrogenation were identified in this work too. In contrast, when the reaction is catalyzed by the same FLP, the activity is solely governed by the steric hindrance of the alkynes. The overall activity exhibits a volcano-shaped trend as a function of the buried volume of the alkynes. FLP (d1) shows the highest activity toward hex-3-yne, as predicted by the volcano correlation, while it exhibits lower activity toward alkynes with either less or greater steric hindrance compared to hex-3-yne.
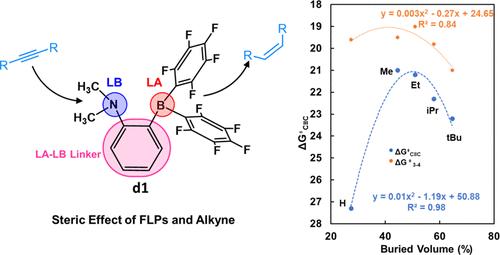
受挫路易斯对催化炔半氢化的立体效应理论研究
受挫路易斯对(FLPs)为小分子的活化和转化提供了一种独特的途径,因而受到越来越多的关注。在此,我们利用密度泛函计算研究了 FLPs 和炔烃对炔烃半氢化活性的电子和立体效应,炔烃半氢化是生产药物和聚合物的关键反应。我们将模型催化剂 FLP(1-NMe2-2-B(C6F5)2-C6H4 (d1))的 LA-LB 连接物 (d) 替换为各种连接物,从而研究了 FLP 的立体效应。此外,我们还研究了炔烃的立体效应,将底物从乙炔改为丁-2-炔、己-3-炔、2,5-二甲基己-3-炔、2,2,5,5-四甲基己-3-炔和 1,2-二苯基乙炔。我们的计算结果表明,在使用具有不同 LA-LB 连接物的氟烯烃时,炔和烯烃氢化的整体活性受到电子和立体效应的影响。为了获得更好的活性,可以增加 LA 邻近环境的立体阻碍,降低 FLPs LB 位点的电子密度。这项工作还确定了涉及炔烃半氢化的每个基本步骤的结构-活性关系。与此相反,当反应由相同的 FLP 催化时,活性完全受炔烃的立体阻碍作用的影响。整体活性与炔烃的埋藏量呈火山状变化。正如火山相关性所预测的那样,FLP(d1)对己-3-炔的活性最高,而对立体阻碍比己-3-炔小或大的炔烃的活性较低。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


