Preparation of Bi2CrO6/CuO heterostructure nanocomposite to increase methylene blue decomposition under visible light irradiation
IF 4.3
3区 材料科学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
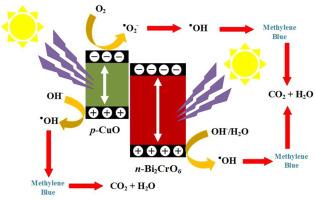
In this study, the Bi2CrO6/CuO nanocomposite was used for the photocatalytic degradation of methylene blue (MB) under visible light. Nanocomposites with different ratios (1:1, 1:2, 2:1) were synthesized under hydrothermal conditions and investigated for MB removal. Various characterization techniques, including XRD, FT-IR, FE-SEM, BET, DRS, PL, and EDS, were employed to elucidate the physicochemical aspects of the catalysts. The 2:1 nanocomposite ratio was selected as the photocatalyst with the highest removal efficiency (85 %). Four main factors, including initial concentration, solution pH, catalyst dose, and light intensity, were studied using the response surface methodology (RSM) and the Box-Behnken model. Under optimal conditions, the MB removal efficiency reached 90.06 %. Additionally, the effect of oxidizing agents (H2O2) on the enhanced removal of MB was investigated. The improved photocatalytic performance of the Bi2CrO6/CuO (2:1) nanocomposite is attributed to visible light absorption, the formation of a p-n heterostructure, efficient charge separation via the S-scheme mechanism, and increased charge carrier lifetime. Moreover, economic calculations showed that the estimated costs for the photocatalytic removal of MB from wastewater are cost-effective.
制备 Bi2CrO6/CuO 异质结构纳米复合材料,提高可见光照射下亚甲基蓝的分解率
本研究利用 Bi2CrO6/CuO 纳米复合材料在可见光下光催化降解亚甲基蓝(MB)。在水热条件下合成了不同比例(1:1、1:2、2:1)的纳米复合材料,并研究了其去除甲基溴的性能。采用了各种表征技术,包括 XRD、FT-IR、FE-SEM、BET、DRS、PL 和 EDS,以阐明催化剂的物理化学方面。结果表明,2:1 纳米复合比例的光催化剂具有最高的去除效率(85%)。利用响应面方法(RSM)和箱-贝肯模型研究了四个主要因素,包括初始浓度、溶液 pH 值、催化剂剂量和光照强度。在最佳条件下,甲基溴的去除率达到了 90.06%。此外,还研究了氧化剂(H2O2)对提高甲基溴去除率的影响。Bi2CrO6/CuO (2:1) 纳米复合材料光催化性能的提高归因于对可见光的吸收、p-n 异质结构的形成、通过 S 型机制实现的高效电荷分离以及电荷载流子寿命的延长。此外,经济计算表明,光催化去除废水中甲基溴的估计成本具有成本效益。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Chemistry and Physics
工程技术-材料科学:综合
CiteScore
8.70
自引率
4.30%
发文量
1515
审稿时长
69 days
期刊介绍:
Materials Chemistry and Physics is devoted to short communications, full-length research papers and feature articles on interrelationships among structure, properties, processing and performance of materials. The Editors welcome manuscripts on thin films, surface and interface science, materials degradation and reliability, metallurgy, semiconductors and optoelectronic materials, fine ceramics, magnetics, superconductors, specialty polymers, nano-materials and composite materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


