A comprehensive molecular description of sertraline hydrochloride: From solid state to electronic structure
IF 3
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
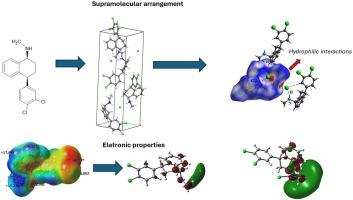
Sertraline is a selective serotonin reuptake inhibitor (SSRI) widely used as an antidepressant. The presence of HCl in forming sertraline hydrochloride affects its physicochemical properties. This article aimed to understand the molecular and electronic structures of sertraline hydrochloride. For this, theoretical calculations were carried out at the DFT/M06-2X/6-311++G(d,p) level of theory. The molecular topology was studied. In the case of the C10–H⋯Cl interaction, the Hirshfeld surface showed that the contacts between the H atom and the Cl anion equal the sum of the van der Waals radii. This structure is susceptible to electrophilic attacks according to the Fukui function. Frontier molecular orbital (HOMO and LUMO) showed that the presence of HCl increased the acidic character of the sertraline. Topological analysis of SERTH showed that N![]() H⋯Cl hydrogen bond type interactions are predominant and contribute to the stability of the crystal.
H⋯Cl hydrogen bond type interactions are predominant and contribute to the stability of the crystal.
盐酸舍曲林的全面分子描述:从固态到电子结构
舍曲林是一种选择性血清素再摄取抑制剂(SSRI),被广泛用作抗抑郁药。盐酸舍曲林中盐酸的存在会影响其理化性质。本文旨在了解盐酸舍曲林的分子结构和电子结构。为此,我们在 DFT/M06-2X/6-311++G(d,p) 理论水平上进行了理论计算。对分子拓扑结构进行了研究。在 C10-H⋯Cl 相互作用的情况下,Hirshfeld 表面显示 H 原子和 Cl 阴离子之间的接触等于范德华半径之和。根据 Fukui 函数,这种结构容易受到亲电攻击。前沿分子轨道(HOMO 和 LUMO)显示,盐酸的存在增加了舍曲林的酸性。SERTH 的拓扑分析表明,NH⋯Cl 氢键型相互作用占主导地位,并有助于晶体的稳定性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Computational and Theoretical Chemistry
CHEMISTRY, PHYSICAL-
CiteScore
4.20
自引率
10.70%
发文量
331
审稿时长
31 days
期刊介绍:
Computational and Theoretical Chemistry publishes high quality, original reports of significance in computational and theoretical chemistry including those that deal with problems of structure, properties, energetics, weak interactions, reaction mechanisms, catalysis, and reaction rates involving atoms, molecules, clusters, surfaces, and bulk matter.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


