First-principles density functional study of iodine molecule adsorption on stable CuS surfaces
IF 3
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The present work investigated the adsorption of gaseous iodine molecules (I2) on stable CuS surface, which has demonstrated excellent performance as an adsorbent for I2 removal, with first-principles density functional theory (DFT). In this work, a pair of asymmetric surfaces (marked as slab1 and slab2) formed by breaking the weakest bond along (0 0 1) direction are chosen to present CuS surfaces. The findings indicate that the adsorption of I2 molecules on the pristine CuS(0 0 1) surface is relatively weak, while surface defects significantly enhance the binding strength of I2. In particular, S-vacancy CuS(0 0 1) surfaces exhibit considerably higher adsorption energy for I2 compared to Cu-vacancy surfaces. We found that the hollow and Cu-top sites are typically the dominant adsorption sites, and the initial orientation of I2 relative to the surface also influences the adsorption performance.
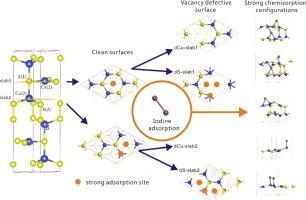
稳定 CuS 表面碘分子吸附的第一原理密度泛函研究
稳定的 CuS 表面作为去除 I2 的吸附剂表现出卓越的性能,本研究采用第一原理密度泛函理论(DFT)研究了气态碘分子(I2)在稳定的 CuS 表面上的吸附。在这项研究中,选择了沿 (0 0 1) 方向断裂最弱键而形成的一对不对称表面(标记为 slab1 和 slab2)来呈现 CuS 表面。研究结果表明,I2 分子在原始 CuS(0 0 1)表面上的吸附相对较弱,而表面缺陷则能显著增强 I2 的结合强度。特别是,与 Cu 真空表面相比,S 真空 CuS(0 0 1) 表面对 I2 的吸附能要高得多。我们发现,空心位和 Cu 顶位点通常是主要的吸附位点,I2 相对于表面的初始取向也会影响吸附性能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Computational and Theoretical Chemistry
CHEMISTRY, PHYSICAL-
CiteScore
4.20
自引率
10.70%
发文量
331
审稿时长
31 days
期刊介绍:
Computational and Theoretical Chemistry publishes high quality, original reports of significance in computational and theoretical chemistry including those that deal with problems of structure, properties, energetics, weak interactions, reaction mechanisms, catalysis, and reaction rates involving atoms, molecules, clusters, surfaces, and bulk matter.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


