Solution and solid state structures of lanthanides(III) and uranyl(II) complexes of tripodal tris(2-pyridyl)-containing ligand on Ph3P(O) platform
IF 2.4
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
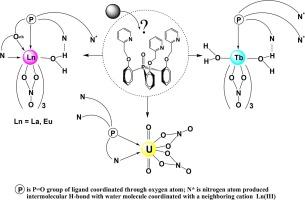
Reaction of tris[2-(2′-pyridylmethoxy)phenyl]phosphine oxide (L) with f-element nitrates resulted in 1:1 complexes. The isolated complexes of ligand L with La(III), Eu(III), Tb(III), and U(VI) nitrates, and with La(III) chloride were studied in the solid state and solution by IR, Raman, and NMR spectroscopy, X-ray analysis, and also DFT calculations. Composition and structure of the complexes vary with lanthanide cation radius. According to the data of elemental analysis, vibrational spectroscopy, and X-ray diffraction, the ligand is coordinated in tridentate mode in crystalline La(III) and solid Eu(III) complexes: [Ln(OPO,N,Oeth-L)(H2O)(O,O-NO3)3], and in monodentate mode in crystalline complex [Tb(OPO-L)(H2O)2(O,O-NO3)3]. Nitrogen atoms of pyridine fragments of the ligand not involved in coordination produce intra- and intermolecular H-bonds with coordinated water molecules in the second coordination sphere of Ln(III). According to IR, NMR spectrometry, and DFT calculations, the structure of coordination polyhedron of the main species of Ln(III) complexes in acetonitrile solutions is retained including coordination of one of pyridine fragments in the second coordination sphere: [Ln{OPO,N,(N*),Oeth-L}(H2O)(O,O-NO3)3] and [Tb{OPO,(N*)-L}(H2O)2(O,O-NO3)3], where Ln = La, Eu, and N* is the nitrogen atom of pyridine fragment producing intramolecular H-bond with coordinated water molecule. According IR and Raman spectroscopy, ligand L is coordinated in bidentate mode in solid complex [UO2(OPO,N-L)(O,O-NO3)], and uncoordinated nitrogen atoms remain free. Solution structure of uranyl complex is labile and depends on solvent nature. Equilibria of complex species with variable coordination of nitrate ions, ligand L, and with probable involvement of water take place in CD3CN and CDCl3 solutions. Photophysical properties of the prepared Eu(III) and Tb(III) complexes were studied. The preliminary assessment of extraction properties of compound L was made. Stability of studied compounds in acetonitrile solutions was examined, and the structure of one of protolysis product of Eu(III) complex, [Eu(LH)(H2O)(NO3)4], was established by micro-IR and X-ray analysis.
含三(2-吡啶基)配体的镧系元素(III)和铀酰(II)配合物在 Ph3P(O)平台上的溶液和固态结构
三[2-(2′-吡啶基甲氧基)苯基]氧化膦(L)与 f 元素硝酸盐反应生成 1:1 的络合物。通过红外光谱、拉曼光谱、核磁共振光谱、X 射线分析以及 DFT 计算,研究了配体 L 与 La(III)、Eu(III)、Tb(III) 和 U(VI) 硝酸盐以及 La(III) 氯化物在固态和溶液中的分离配合物。配合物的组成和结构随镧系阳离子半径的变化而变化。根据元素分析、振动光谱和 X 射线衍射数据,配体在结晶 La(III) 和固体 Eu(III) 复合物 [Ln(OPO,N,Oeth-L)(H2O)(O,O-NO3)3] 中以三叉配位模式配位,在结晶复合物 [Tb(OPO-L)(H2O)2(O,O-NO3)3] 中以单配位模式配位。配体中不参与配位的吡啶片段的氮原子与 Ln(III) 第二配位圈中的配位水分子产生分子内和分子间 H 键。根据红外光谱、核磁共振光谱和 DFT 计算,Ln(III) 复合物主要种类在乙腈溶液中的配位多面体结构得以保留,包括其中一个吡啶片段在第二配位层中的配位:[Ln{OPO,N,(N*),Oeth-L}(H2O)(O,O-NO3)3]和[Tb{OPO,(N*)-L}(H2O)2(O,O-NO3)3],其中 Ln = La、Eu,N* 是吡啶片段的氮原子,与配位水分子产生分子内 H 键。根据红外光谱和拉曼光谱,配体 L 在固体配合物 [UO2(OPO,N-L)(O,O-NO3)]中以双配位方式配位,未配位的氮原子保持自由状态。铀酰络合物的溶液结构是易变的,取决于溶剂的性质。在 CD3CN 和 CDCl3 溶液中,硝酸根离子和配体 L 的配位各不相同,而且可能有水的参与。研究了制备的 Eu(III)和 Tb(III)配合物的光物理特性。对化合物 L 的萃取特性进行了初步评估。考察了所研究化合物在乙腈溶液中的稳定性,并通过显微红外和 X 射线分析确定了 Eu(III)络合物的一种原分解产物[Eu(LH)(H2O)(NO3)4]的结构。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Polyhedron
化学-晶体学
CiteScore
4.90
自引率
7.70%
发文量
515
审稿时长
2 months
期刊介绍:
Polyhedron publishes original, fundamental, experimental and theoretical work of the highest quality in all the major areas of inorganic chemistry. This includes synthetic chemistry, coordination chemistry, organometallic chemistry, bioinorganic chemistry, and solid-state and materials chemistry.
Papers should be significant pieces of work, and all new compounds must be appropriately characterized. The inclusion of single-crystal X-ray structural data is strongly encouraged, but papers reporting only the X-ray structure determination of a single compound will usually not be considered. Papers on solid-state or materials chemistry will be expected to have a significant molecular chemistry component (such as the synthesis and characterization of the molecular precursors and/or a systematic study of the use of different precursors or reaction conditions) or demonstrate a cutting-edge application (for example inorganic materials for energy applications). Papers dealing only with stability constants are not considered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


