Melatonin protects against particulate matter-induced ovarian dysfunction by activating the Nrf2 signaling pathway to alleviate ferroptosis
IF 5.2
2区 医学
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
Abstract
Accumulating evidence suggests that exposure to ambient airborne PM2.5 increases the risk of primary ovarian insufficiency (POI). However, whether ferroptosis, a newly discovered type of cell death involved in PM2.5-induced lung injury and fibrosis, is involved in PM2.5-induced POI has not been determined. This study aimed to verify the involvement of PM2.5-induced ferroptosis in ovarian dysfunction and further demonstrate that melatonin inhibits ferroptosis by activating the Nrf2 signaling pathway to ameliorate POI in vivo and in vitro. In our study, PM2.5 promoted iron accumulation and induced lipid peroxidation, thus contributing to ferroptosis in KGN cells and ovaries. However, these effects were eliminated and enhanced in Nrf2-overexpressing and Nrf2-knockdown cells, respectively. In addition, melatonin and ferrostatin-1 (Fer-1) inhibited ferroptosis by activating the NRF2 signaling pathway, as evidenced by the silencing of Nrf2 in vivo and in vitro. Mechanistically, Nrf2-knockout mice were more susceptible to ferroptosis and PM2.5-induced POI than control mice. Moreover, melatonin suppressed changes in morphological and biochemical indicators related to ferroptosis, such as MDA and GSH depletion and GPX4 and XCT downregulation, by enhancing Nrf2 signaling. Here, we first reported that PM2.5 triggered ferroptosis by increasing ROS levels, lipid peroxidation and glutathione depletion. Notably, melatonin significantly decreased ferroptosis levels and improved ovarian function by activating the NRF2 signaling pathway in vivo and in vitro.
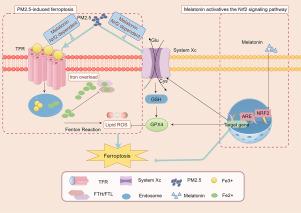
褪黑素通过激活Nrf2信号通路来缓解铁氧化,从而保护卵巢免受微粒物质诱发的卵巢功能障碍的影响。
越来越多的证据表明,暴露于环境空气中的 PM2.5 会增加原发性卵巢功能不全(POI)的风险。然而,新发现的参与PM2.5诱导的肺损伤和肺纤维化的细胞死亡类型--铁蛋白沉积是否参与了PM2.5诱导的卵巢功能不全尚未确定。本研究旨在验证PM2.5诱导的铁凋亡参与了卵巢功能障碍,并进一步证明褪黑素可通过激活Nrf2信号通路抑制铁凋亡,从而改善体内和体外的POI。在我们的研究中,PM2.5 促进了铁的积累并诱导了脂质过氧化,从而导致了 KGN 细胞和卵巢中的铁突变。然而,这些影响在Nrf2-缺失表达细胞和Nrf2-敲除细胞中分别被消除和增强。此外,褪黑素和铁前列素-1(Fer-1)通过激活NRF2信号通路抑制铁猝死,体内和体外的Nrf2沉默也证明了这一点。从机制上讲,与对照组小鼠相比,Nrf2-基因敲除小鼠更容易发生铁蛋白沉积和PM2.5诱导的POI。此外,褪黑素通过增强 Nrf2 信号传导,抑制了与铁败坏相关的形态学和生化指标的变化,如 MDA 和 GSH 的耗竭以及 GPX4 和 XCT 的下调。在这里,我们首次报道了PM2.5通过增加ROS水平、脂质过氧化和谷胱甘肽耗竭引发铁变态反应。值得注意的是,褪黑素通过激活体内和体外的 NRF2 信号通路,明显降低了铁蛋白沉积水平并改善了卵巢功能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Life sciences
医学-药学
CiteScore
12.20
自引率
1.60%
发文量
841
审稿时长
6 months
期刊介绍:
Life Sciences is an international journal publishing articles that emphasize the molecular, cellular, and functional basis of therapy. The journal emphasizes the understanding of mechanism that is relevant to all aspects of human disease and translation to patients. All articles are rigorously reviewed.
The Journal favors publication of full-length papers where modern scientific technologies are used to explain molecular, cellular and physiological mechanisms. Articles that merely report observations are rarely accepted. Recommendations from the Declaration of Helsinki or NIH guidelines for care and use of laboratory animals must be adhered to. Articles should be written at a level accessible to readers who are non-specialists in the topic of the article themselves, but who are interested in the research. The Journal welcomes reviews on topics of wide interest to investigators in the life sciences. We particularly encourage submission of brief, focused reviews containing high-quality artwork and require the use of mechanistic summary diagrams.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


