Synthesis and biological evaluation of sugar-modified truncated carbanucleosides as A2A and A3 adenosine receptor ligands to explore conformational effect to the receptors
IF 3.3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
This study investigated the impact of conformation on the binding affinity of carbanucleosides to A2A and A3 adenosine receptors (ARs). A series of nucleosides, including saturated, unsaturated, North (N)-methano, and South (S)-methanocarbanucleosides was prepared, and their binding affinities to A2AAR and A3AR were assessed. Biological evaluations revealed that all synthesized (S)-methanocarbanucleosides had negligible binding to both receptors, and most (N)-methanocarbanucleosides exhibited high binding affinities. Molecular docking analysis showed that the (N)-methanocarbanucleoside 6a exhibited favorable interactions and minimal steric clashes in both A2AAR and A3AR. Conversely, the (S)-methanocarbanucleoside 7a appears to encounter significant steric clashes, which impeded its binding to A2AAR. Furthermore, when adopting the South conformation 7a was unable to bind to A3AR. Expanding upon the (N)-methanocarba moiety, various C8-aromatic groups were introduced to convert A2AAR agonists into antagonists and these modified compounds also exhibited strong binding affinity. These results suggest that the North conformation is favored by both A2AAR and A3AR, and that (N)-methanocarbanucleosides can serve as versatile structural moieties for dual targeting of A2AAR and A3AR. These findings offer promising avenues for the development of dual ligands for therapeutic applications in obesity and immunotherapy.
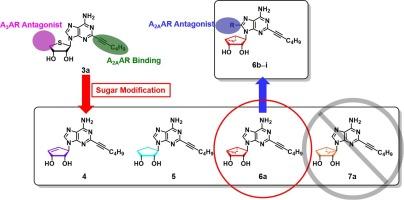
糖修饰的截短碳核苷作为 A2A 和 A3 腺苷受体配体的合成和生物学评价,以探索对受体的构象效应。
本研究探讨了构象对碳核苷与A2A和A3腺苷受体(ARs)结合亲和力的影响。研究人员制备了一系列核苷,包括饱和、不饱和、北(N)-甲基和南(S)-甲基卡巴核苷,并评估了它们与 A2AAR 和 A3AR 的结合亲和力。生物学评价结果表明,所有合成的(S)-甲氰基巴豆核苷与这两种受体的结合力都微乎其微,而大多数(N)-甲氰基巴豆核苷都表现出很高的结合亲和力。分子对接分析表明,(N)-甲基巴豆核苷 6a 在 A2AAR 和 A3AR 中均表现出有利的相互作用和最小的立体冲突。相反,(S)-甲基巴豆核苷 7a 似乎遇到了严重的立体冲突,阻碍了它与 A2AAR 的结合。此外,当采用南构象时,7a 无法与 A3AR 结合。在 (N)-methanocarba 分子的基础上,引入了各种 C8 芳香基团,将 A2AAR 激动剂转化为拮抗剂,这些修饰后的化合物也表现出很强的结合亲和力。这些结果表明,北构象同时受到 A2AAR 和 A3AR 的青睐,(N)-甲氧羰基核苷可作为 A2AAR 和 A3AR 双靶向的多功能结构分子。这些发现为开发治疗肥胖症和免疫疗法的双配体提供了广阔的前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


