Knockdown of ANO1 decreases TGF-β- and IL-6-induced adhesion and migration of cardiac fibroblasts by inhibiting the expression of integrin and focal adhesion kinase
IF 3.3
3区 生物学
Q3 CELL BIOLOGY
引用次数: 0
Abstract
Ischemic cardiac injury triggers a significant inflammatory response, activating and mobilizing cardiac fibroblasts (CFs), which ultimately contributes to myocardial fibrosis. In this study, we investigated the role of ANO1, a calcium-activated chloride channel (CaCC) protein, in regulating CFs migration and adhesion under inflammatory conditions. Our results demonstrated that ANO1 knockdown significantly attenuated TGF-β- and IL-6-induced adhesion and migration of CFs. This inhibitory effect was mediated through the downregulation of integrin expression and reduced activation of focal adhesion kinase (FAK), key components in cellular adhesion and motility pathways. This study provides new insights into the mechanisms underlying CFs migration and adhesion, highlighting the potential of ANO1 as a therapeutic target for mitigating adverse fibrotic remodeling following myocardial infarction.
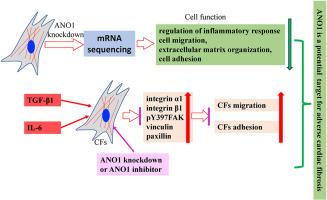
通过抑制整合素和局灶粘附激酶的表达,敲除 ANO1 可降低 TGF-β 和 IL-6 诱导的心脏成纤维细胞的粘附和迁移。
缺血性心脏损伤会引发严重的炎症反应,激活并动员心脏成纤维细胞(CFs),最终导致心肌纤维化。在这项研究中,我们研究了钙激活氯通道(CaCC)蛋白 ANO1 在炎症条件下调控成纤维细胞迁移和粘附的作用。结果表明,敲除 ANO1 能明显减弱 TGF-β 和 IL-6 诱导的 CFs 粘附和迁移。这种抑制作用是通过下调整合素表达和减少激活细胞粘附和迁移通路的关键成分--局灶粘附激酶(FAK)来介导的。这项研究为了解CFs迁移和粘附的机制提供了新的视角,凸显了ANO1作为治疗靶点减轻心肌梗死后不良纤维重塑的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental cell research
医学-细胞生物学
CiteScore
7.20
自引率
0.00%
发文量
295
审稿时长
30 days
期刊介绍:
Our scope includes but is not limited to areas such as: Chromosome biology; Chromatin and epigenetics; DNA repair; Gene regulation; Nuclear import-export; RNA processing; Non-coding RNAs; Organelle biology; The cytoskeleton; Intracellular trafficking; Cell-cell and cell-matrix interactions; Cell motility and migration; Cell proliferation; Cellular differentiation; Signal transduction; Programmed cell death.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


