Zhuangyao Jianshen Wan ameliorates senile osteoporosis in SAMP6 mice through Modulation of the GCN5L1-mediated PI3K/Akt/wnt signaling pathway
IF 5.9
1区 医学
Q1 ORTHOPEDICS
引用次数: 0
Abstract
Background
Senile osteoporosis (SOP) is a systemic bone disease characterized by increased susceptibility to fractures. However, there is currently no effective treatment for SOP. The Zhuangyao Jianshen Wan (ZYJSW) pill is traditionally believed to possess kidney-nourishing and bone-strengthening effects, demonstrating efficacy in treating fractures. Despite this, its effectiveness and mechanism in SOP remain unclear. This study aims to investigate the therapeutic potential of ZYJSW in treating SOP in senescence accelerated mouse prone 6 (SAMP6, P6) mice, and elucidate the underlying mechanisms.
Methods
Four-month-old SAMP6 mice were categorized into six groups: the model group (SAMP6), low, medium, and high-dose ZYJSW treatment groups, calcitriol treatment (positive control 1) group, and metformin treatment (positive control 2) group. Gastric administration was carried out for 15 weeks, and a normal control group comprising four-month-old Senescence-Accelerated Mouse Resistant 1 (SAMR1) mice. Changes in body weight, liver and kidney function, bone protective effects, and muscle quality were evaluated using various assays, including H&E staining, Goldner staining, bone tissue morphology analysis, Micro-CT imaging, and biomechanical testing. Qualitative analysis and quality control of ZYJSW were performed via LC-MS/MS analysis. To explore mechanisms, network pharmacology and proteomics were employed, and the identified proteins were validated by Western blotting.
Results
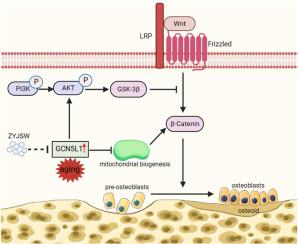
Oral administration of ZYJSW to P6 mice exerted preventive efficacy against osteopenia, impaired bone microstructure, and poor bone and muscle quality. ZYJSW attenuated the imbalance in bone metabolism by promoting bone formation, as evidenced by the upregulation of key factors such as Runt-related transcription factor 2 (RUNX2), Bone Morphogenetic Protein (BMP2), Osteoprotegerin (OPG) and Osteocalcin (OCN), while simultaneously inhibiting bone resorption through the downregulation of TNF receptor associated factor 6 (TRAF6), Tartrate resistant acid phosphatase (TRAP), Receptor activator for nuclear factor-κB ligand (RANKL) and Cathepsin K (CTSK). Additionally, ZYJSW enhanced muscle structure and function by counteracting the elevation of Ubiquitin (Ub), Muscle RING-finger protein-1 (Murf-1), F-Box Protein 32 (FBOX32), and Myogenin (Myog). Network pharmacology predictions, proteomics analysis corroborated by published literature demonstrated the role of ZYJSW involving in safeguarding mitochondrial biogenesis. This was achieved by suppressing GCN5L1 expression, contributing to the heightened expression of TFAM, PGC-1α, and nuclear respiratory factor-1 (NRF-1) proteins. ZYJSW also positively modulated Wnt signaling pathways responsible for bone formation, due to regulating expressions of key components like β-catenin, GSK-3β, and LRP5. In addition, ZYJSW causes the downregulation of the PI3K/Akt pathway by inhibiting the phosphorylation of both PI3K and Akt.
Conclusions
The study highlights the significance of ZYJSW in preserving the health of both bone and muscle in P6 mice, potentially through the regulation of the GCN5L1-mediated PI3K/Akt/Wnt signaling pathway.
The translational potential of this article
Our research provides evidence and a mechanistic rationale for ZYJSW as a candidate for SOP treatment, offering insights for further exploration and strategy development.
壮骨药丸通过调节 GCN5L1 介导的 PI3K/Akt/wnt 信号通路改善 SAMP6 小鼠的老年性骨质疏松症
背景梅毒性骨质疏松症(SOP)是一种全身性骨病,其特点是更容易发生骨折。然而,目前尚无有效的治疗方法。传统医学认为,补肾壮骨丸具有补肾壮骨的功效,对治疗骨折有一定疗效。尽管如此,其对澳门巴黎人娱乐官网的疗效和机制仍不明确。方法将四个月大的 SAMP6 小鼠分为六组:模型组(SAMP6)、低、中、高剂量 ZYJSW 治疗组、钙三醇治疗组(阳性对照 1)和二甲双胍治疗组(阳性对照 2)。胃给药组为期 15 周,正常对照组由四个月大的衰老加速小鼠抗性 1(SAMR1)小鼠组成。通过各种检测方法,包括 H&E 染色、Goldner 染色、骨组织形态分析、Micro-CT 成像和生物力学测试,对体重、肝肾功能、骨保护作用和肌肉质量的变化进行了评估。通过 LC-MS/MS 分析对 ZYJSW 进行了定性分析和质量控制。结果给 P6 小鼠口服 ZYJSW 对骨质增生、骨微结构受损以及骨和肌肉质量差具有预防作用。ZYJSW 可促进骨形成,从而减轻骨代谢失衡,这体现在 Runt 相关转录因子 2 (RUNX2)、骨形态发生蛋白 (BMP2)、骨蛋白激酶 (OPG) 和骨钙素 (OCN) 等关键因子的上调、同时通过下调 TNF 受体相关因子 6(TRAF6)、酒石酸抗性酸性磷酸酶(TRAP)、核因子κB 配体受体激活剂(RANKL)和酪蛋白酶 K(CTSK)抑制骨吸收。此外,ZYJSW 还能抵消泛素(Ub)、肌肉环指蛋白-1(Murf-1)、F-Box 蛋白 32(FBOX32)和肌原蛋白(Myog)的升高,从而增强肌肉的结构和功能。网络药理学预测、蛋白质组学分析以及已发表的文献证实了 ZYJSW 在保护线粒体生物生成方面的作用。这一作用是通过抑制 GCN5L1 的表达来实现的,从而促进了 TFAM、PGC-1α 和核呼吸因子-1(NRF-1)蛋白的表达。ZYJSW 还能积极调节负责骨形成的 Wnt 信号通路,这是因为它能调节 β-catenin、GSK-3β 和 LRP5 等关键成分的表达。此外,ZYJSW 还能抑制 PI3K 和 Akt 的磷酸化,从而导致 PI3K/Akt 通路的下调。结论这项研究强调了 ZYJSW 对保护 P6 小鼠骨骼和肌肉健康的重要意义,它可能是通过调节 GCN5L1 介导的 PI3K/Akt/Wnt 信号通路来实现的。本文的转化潜力我们的研究为 ZYJSW 作为 SOP 治疗候选药物提供了证据和机理依据,为进一步探索和制定策略提供了启示。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Orthopaedic Translation
Medicine-Orthopedics and Sports Medicine
CiteScore
11.80
自引率
13.60%
发文量
91
审稿时长
29 days
期刊介绍:
The Journal of Orthopaedic Translation (JOT) is the official peer-reviewed, open access journal of the Chinese Speaking Orthopaedic Society (CSOS) and the International Chinese Musculoskeletal Research Society (ICMRS). It is published quarterly, in January, April, July and October, by Elsevier.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


