Molecular modeling aided design, synthesis, and activity evaluation of N-arylindole derivatives as GPR52 agonists
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
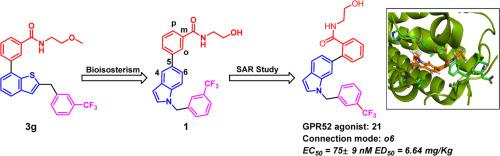
G protein-coupled receptor 52 (GPR52) is considered to be a promising target to improve the symptoms of psychiatric disorders and its agonists are expected to treat schizophrenia without traditional side effects. Several research institutions have reported some small molecule GPR52 agonists, which can be the starting point for rational drug development. In this study, a series of N-arylindole derivatives were designed and synthesized based on 3g according to classical pharmacochemical methods and computer aided drug design (CADD). The designed compounds exhibited good to excellent activities and the structure-activity relationship (SAR) study was explored. The results show that the connection mode between the hydrophilic head (Part I) and the indole ring (Part II) plays an important role in the GPR52 agonist activity. Among these compounds, compounds 16 and 21 have good GPR52 agonist activity (EC50 = 93 nM and 75 nM) and can inhibit hyperactive behavior in mice induced by MK-801 (EC50 =7.94 mg/kg and 6.64 mg/kg). These designed small molecules will provide new options for the development of novel GPR52 agonists.
分子建模辅助设计、合成和评估作为 GPR52 激动剂的 N-芳基吲哚衍生物的活性
G 蛋白偶联受体 52(GPR52)被认为是一个很有希望改善精神疾病症状的靶点,其激动剂有望治疗精神分裂症而不会产生传统的副作用。一些研究机构已经报道了一些小分子 GPR52 激动剂,它们可以作为合理药物开发的起点。本研究根据经典的药物化学方法和计算机辅助药物设计(CADD),在3g的基础上设计并合成了一系列N-芳基吲哚衍生物。所设计的化合物表现出良好至卓越的活性,并对其进行了结构-活性关系(SAR)研究。结果表明,亲水头(第一部分)和吲哚环(第二部分)之间的连接模式在 GPR52 激动剂活性中起着重要作用。在这些化合物中,化合物 16 和 21 具有良好的 GPR52 激动剂活性(EC50 = 93 nM 和 75 nM),并能抑制 MK-801 诱导的小鼠多动行为(EC50 = 7.94 mg/kg 和 6.64 mg/kg)。这些设计的小分子将为开发新型 GPR52 激动剂提供新的选择。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


