Synthesis, general physicochemical behaviour and magnetic studies of the macrocyclic bis-pyridine derivatives [TTDPzM(py)2]·2H2O (M = MnII, CoII, NiII)
IF 2.7
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
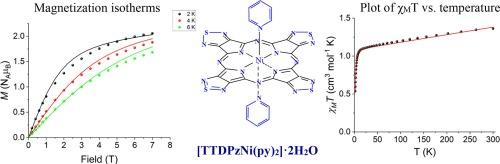
In our extensive work on phthalocyanine-like macrocycles carrying peripherally attached strongly electron-withdrawing 1,2,5-thia/selenodiazol rings we recently reported on the FeII species of formula [TTDPzFe(py)2].2H2O (TTDPz = tetrakis(thiadiazole)porphyrazinato dianion). The present work deals with the synthesis and extended physicochemical studies of the series of related macrocycles [TTDPzM(py)2].2H2O (M = MnII, CoII, NiII). The diffractograms of three solid samples of the NiII complex [TTDPzNi(py)2]·2H2O, obtained from different synthetic procedures, indicate that the species is reproducibly obtained. All three samples are isomorphous with each other as shown by their X-ray powder diffractograms. Based on the comparison of the IR spectra of [TTDPzNi(py)2].2H2O and the related desolvated [TTDPzNi], the IR absorption peaks belonging to the pyridine molecules could be identified. The systematic presence of the two water molecules in all three species suggests that forms of contact do exist in the form of hydrogen bonds between them and the S and N atoms present peripherally in the thiadiazole groups of the macrocyclic units. As regard to the elimination of water, the thermograms (in inert atmosphere in the range 25–600 °C) indicate in all cases that loss of water occurs below 100 °C but loss of pyridine occurs over a broad range 100–300 °C for the MnII and CoII species whereas for the NiII complex it takes place in the narrow range 200–220 °C. In relation to the parallel series of metallophthalocyanines, i.e. [PcM] (M = MnII, FeII, CoII), it is noted that the NiII analog has not been reported in the literature and in a direct experiment it has been proved that [PcNi] heated in pyridine is obtained unchanged, the different behavior for the NiII TTDPz derivative due to the electron withdrawing effect of the external thiadiazole substituents, which makes favorable axial pyridine coordination in the complex. Completely insoluble in water, the triad of TTDPz derivatives is extremely low-soluble in non-donor or low donor solvents. This precluded any attempt to isolate single crystals for single-crystal X-ray work. Their UV–visible spectra show essential similarities in whatever organic solvent is used with clearly positioned Soret (300–400 nm) and Q-band absorptions (630–650 nm). Variable temperature magnetic susceptibility and magnetization studies reveal that the [TTDPzM(py)2].2H2O complexes all show paramagnetism with ground state spins of 3/2 (MnII), ½ (CoII) and 1 (NiII), supported by fitting the data to spin Hamiltonian formalism, vide infra. Comparisons are made with the magnetism of [PcM] analogues with key differences noted for M = NiII.
大环双吡啶衍生物 [TTDPzM(py)2]-2H2O(M = MnII、CoII、NiII)的合成、一般物理化学行为和磁性研究
我们对酞菁类大环进行了大量研究,这些大环外围附有强吸电子的 1,2,5-噻二唑/硒二唑环,最近我们报告了式 [TTDPzFe(py)2].2H2O(TTDPz = 四(噻二唑)卟嗪二元离子)的 FeII 物种。本研究涉及一系列相关大环[TTDPzM(py)2].2H2O(M = MnII、CoII、NiII)的合成和扩展物理化学研究。通过不同合成过程获得的 NiII 复合物 [TTDPzNi(py)2]-2H2O 的三个固体样品的衍射图表明,该复合物是可重复获得的。所有三个样品的 X 射线粉末衍射图都显示它们彼此同构。根据[TTDPzNi(py)2].2H2O 和相关脱溶[TTDPzNi]的红外光谱比较,可以确定属于吡啶分子的红外吸收峰。两种水分子在所有三种物质中的系统性存在表明,它们与大环单元噻二唑基团外围的 S 原子和 N 原子之间确实存在氢键形式的接触。至于水的消除,热图(在 25-600 ℃ 的惰性气氛中)显示,在所有情况下,水的损失都发生在 100 ℃ 以下,但对于 MnII 和 CoII 物种,吡啶的损失发生在 100-300 ℃ 的宽范围内,而对于 NiII 复合物,则发生在 200-220 ℃ 的窄范围内。关于平行系列的金属酞菁,即 [PcM](M = MnII、FeII、CoII),我们注意到文献中还没有报道过 NiII 类似物,在直接实验中证明,[PcNi]在吡啶中加热后不会发生变化,而 NiII TTDPz 衍生物的不同行为则是由于外部噻二唑取代基的退电子效应,这有利于络合物中吡啶的轴向配位。TTDPz 衍生物的三元组完全不溶于水,在非供体或低供体溶剂中的溶解度极低。这就排除了分离单晶进行单晶 X 射线研究的任何尝试。无论使用何种有机溶剂,它们的紫外-可见光谱都显示出基本的相似性,具有清晰的索雷特(300-400 nm)和 Q 波段吸收(630-650 nm)。变温磁感应强度和磁化率研究表明,[TTDPzM(py)2].2H2O 复合物都显示出顺磁性,基态自旋分别为 3/2(锰Ⅱ)、1/2(钴Ⅱ)和 1(镍Ⅱ)。与 [PcM] 类似物的磁性进行了比较,并指出了 M = NiII 的主要差异。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganica Chimica Acta
化学-无机化学与核化学
CiteScore
6.00
自引率
3.60%
发文量
440
审稿时长
35 days
期刊介绍:
Inorganica Chimica Acta is an established international forum for all aspects of advanced Inorganic Chemistry. Original papers of high scientific level and interest are published in the form of Articles and Reviews.
Topics covered include:
• chemistry of the main group elements and the d- and f-block metals, including the synthesis, characterization and reactivity of coordination, organometallic, biomimetic, supramolecular coordination compounds, including associated computational studies;
• synthesis, physico-chemical properties, applications of molecule-based nano-scaled clusters and nanomaterials designed using the principles of coordination chemistry, as well as coordination polymers (CPs), metal-organic frameworks (MOFs), metal-organic polyhedra (MPOs);
• reaction mechanisms and physico-chemical investigations computational studies of metalloenzymes and their models;
• applications of inorganic compounds, metallodrugs and molecule-based materials.
Papers composed primarily of structural reports will typically not be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


