SARS-CoV-2 disrupts host gene networks: Unveiling key hub genes as potential therapeutic targets for COVID-19 management
IF 7
2区 医学
Q1 BIOLOGY
引用次数: 0
Abstract
Purpose
Although the end of COVID-19 as a public health emergency was declared on May 2023, still new cases of the infection are reported and the risk remains of new variants emerging that may cause new surges in cases and deaths. While clinical symptoms have been rapidly defined worldwide, the basic body responses and pathogenetic mechanisms acting in patients with SARS-CoV-2 infection over time until recovery or death require further investigation. The understanding of the molecular mechanisms underlying the development and course of the disease is essential in designing effective preventive and therapeutic approaches, and ultimately reducing mortality and disease spreading.
Methods
The current investigation aimed to identify the key genes engaged in SARS-CoV-2 infection. To achieve this goal high-throughput RNA sequencing of peripheral blood samples collected from healthy donors and COVID-19 patients was performed. The resulting sequence data were processed using a wide range of bioinformatics tools to obtain detailed modifications within five transcriptomic phenomena: expression of genes and long non-coding RNAs, alternative splicing, allel-specific expression and circRNA production. The in silico procedure was completed with a functional analysis of the identified alterations.
Results
The transcriptomic analysis revealed that SARS-CoV-2 has a significant impact on multiple genes encoding ribosomal proteins (RPs). Results show that these genes differ not only in terms of expression but also manifest biases in alternative splicing and ASE ratios. The integrated functional analysis exposed that RPs mostly affected pathways and processes related to infection—COVID-19 and NOD-like receptor signaling pathway, SARS-CoV-2-host interactions and response to the virus. Furthermore, our results linked the multiple intronic ASE variants and exonic circular RNA differentiations with SARS-CoV-2 infection, suggesting that these molecular events play a crucial role in mRNA maturation and transcription during COVID-19 disease.
Conclusions
By elucidating the genetic mechanisms induced by the virus, the current research provides significant information that can be employed to create new targeted therapeutic strategies for future research and treatment related to COVID-19. Moreover, the findings highlight potentially promising therapeutic biomarkers for early risk assessment of critically ill patients.
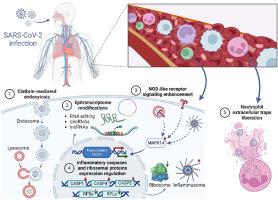
SARS-CoV-2 破坏宿主基因网络:揭示作为 COVID-19 潜在治疗靶点的关键枢纽基因。
目的:尽管 COVID-19 已于 2023 年 5 月被宣布为公共卫生紧急事件,但仍有新的感染病例报告,而且仍有可能出现新的变种,导致新的病例和死亡人数激增。虽然临床症状已在全球范围内迅速确定,但 SARS-CoV-2 感染患者在康复或死亡前的基本身体反应和致病机制还需要进一步研究。了解疾病发生和发展的分子机制对于设计有效的预防和治疗方法,以及最终降低死亡率和疾病传播至关重要:目前的研究旨在确定参与 SARS-CoV-2 感染的关键基因。为实现这一目标,研究人员对从健康捐献者和 COVID-19 患者采集的外周血样本进行了高通量 RNA 测序。利用多种生物信息学工具对测序数据进行处理,以获得五种转录组现象的详细变化:基因和长非编码 RNA 的表达、替代剪接、等位基因特异性表达和 circRNA 的产生。在完成硅学程序的同时,还对已确定的变化进行了功能分析:结果:转录组分析表明,SARS-CoV-2 对编码核糖体蛋白(RPs)的多个基因有重大影响。结果表明,这些基因不仅在表达方面存在差异,而且在替代剪接和 ASE 比例方面也表现出偏差。综合功能分析显示,RPs 主要影响与感染有关的途径和过程--COVID-19 和 NOD 样受体信号途径、SARS-CoV-2-宿主相互作用和对病毒的反应。此外,我们的研究结果还将多种内含子ASE变异和外显子环状RNA分化与SARS-CoV-2感染联系在一起,表明这些分子事件在COVID-19疾病期间的mRNA成熟和转录过程中起着至关重要的作用:通过阐明病毒诱导的遗传机制,目前的研究提供了重要的信息,可用于为 COVID-19 的未来研究和治疗创建新的靶向治疗策略。此外,研究结果还突显了用于危重病人早期风险评估的潜在治疗生物标志物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Computers in biology and medicine
工程技术-工程:生物医学
CiteScore
11.70
自引率
10.40%
发文量
1086
审稿时长
74 days
期刊介绍:
Computers in Biology and Medicine is an international forum for sharing groundbreaking advancements in the use of computers in bioscience and medicine. This journal serves as a medium for communicating essential research, instruction, ideas, and information regarding the rapidly evolving field of computer applications in these domains. By encouraging the exchange of knowledge, we aim to facilitate progress and innovation in the utilization of computers in biology and medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


