Site-Specific Histidine Aza-Michael Addition in Proteins Enabled by a Ferritin-Based Metalloenzyme
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Histidine modifications of proteins are broadly based on chemical methods triggering N-substitution reactions such as aza-Michael addition at histidine’s moderately nucleophilic imidazole side chain. While recent studies have demonstrated chemoselective, histidine-specific modifications by further exploiting imidazole’s electrophilic reactivity to overcome interference from the more nucleophilic lysine and cysteine, achieving site-specific histidine modifications remains a major challenge due to the absence of spatial control over chemical processes. Herein, through X-ray crystallography and cryo-electron microscopy structural studies, we describe the rational design of a nature-inspired, noncanonical amino-acid-incorporated, human ferritin-based metalloenzyme that is capable of introducing site-specific post-translational modifications (PTMs) to histidine in peptides and proteins. Specifically, chemoenzymatic aza-Michael additions on single histidine residues were carried out on eight protein substrates ranging from 10 to 607 amino acids including the insulin peptide hormone. By introducing an insulin-targeting peptide into our metalloenzyme, we further directed modifications to be carried out site-specifically on insulin’s B-chain histidine 5. The success of this biocatalysis platform outlines a novel approach in introducing residue- and, moreover, site-specific post-translational modifications to peptides and proteins, which may further enable reactions to be carried out in vivo.
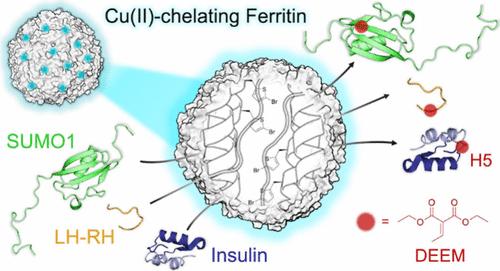
基于铁蛋白的金属酶在蛋白质中实现位点特异性组氨酸氮-迈克尔加成反应
对蛋白质进行组氨酸修饰的化学方法主要是在组氨酸的中等亲核性咪唑侧链上引发 N-取代反应,如氮杂迈克尔加成反应。尽管最近的研究通过进一步利用咪唑的亲电反应性来克服亲核性更强的赖氨酸和半胱氨酸的干扰,证明了化学选择性的组氨酸特异性修饰,但由于缺乏对化学过程的空间控制,实现组氨酸的位点特异性修饰仍然是一项重大挑战。在本文中,我们通过 X 射线晶体学和冷冻电镜结构研究,描述了一种受自然启发的、非典型氨基酸并入的、基于人类铁蛋白的金属酶的合理设计,这种金属酶能够对肽和蛋白质中的组氨酸进行特定位点的翻译后修饰(PTM)。具体而言,在包括胰岛素肽类激素在内的 10 至 607 个氨基酸的 8 种蛋白质底物上,对单个组氨酸残基进行了化学酶促杂合迈克尔添加。通过在我们的金属酶中引入胰岛素靶向肽,我们进一步引导了对胰岛素 B 链组氨酸 5 的位点特异性修饰。这一生物催化平台的成功勾勒出了一种新方法,可对肽和蛋白质进行残基修饰,以及特定位点的翻译后修饰,从而进一步实现体内反应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


