Rapid-response RNA-fluorescence in situ hybridization (FISH) assay platform for coronavirus antiviral high-throughput screening
IF 2.7
4区 生物学
Q2 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
Abstract
Over the past 25 years, the global community has faced challenges posed by three distinct outbreaks of coronaviruses including severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East respiratory syndrome coronavirus (MERS-CoV), and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The identification of a novel alphacoronavirus canine CoV (CCoV-HuPn2018) in human patients in Malaysia underscores the potential for crossover infections to humans. The threat of the ever-evolving nature of viral infections as well as the lingering health and socioeconomic effects of the recent SARS-CoV-2 pandemic emphasize the urgent need for advanced antiviral drug screening tools that can be quickly implemented to strengthen preparedness and preventive measures against future outbreaks. Here, we present the development and validation of a novel RNA-fluorescence in situ hybridization (FISH) imaging assay as a 384-well, high-throughput rapid response platform for antiviral drug discovery. RNA-FISH is a powerful tool to visualize specific mRNA in cultured cells using a high-content imaging platform. The flexibility of RNA-FISH probe sets allows for the rapid design of viral genome-specific probes, enabling in vitro assay development to test for inhibition of viral replication by either biologic or small molecule inhibitors. Screening of 170 antiviral compounds in concentration-response demonstrates a strong correlation between the RNA-FISH assay and an immunofluorescence assay (IFA) for both human coronaviruses HCoV-OC43 and HCoV-229E. Additionally, we successfully applied this methodology in the context of CCoV strain 1–71, proving rapid development and deployment, opening new avenues for the evaluation of antiviral drugs to potential future emerging threats.
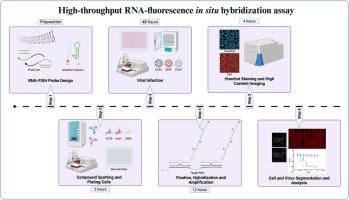
用于冠状病毒抗病毒高通量筛选的快速反应 RNA 荧光原位杂交 (FISH) 检测平台
在过去的 25 年中,全球社会面临着严重急性呼吸系统综合征冠状病毒(SARS-CoV)、中东呼吸系统综合征冠状病毒(MERS-CoV)和严重急性呼吸系统综合征冠状病毒 2(SARS-CoV-2)等三种不同冠状病毒爆发所带来的挑战。在马来西亚的人类患者中发现了一种新型α-冠状病毒犬科 CoV(CCoV-HuPn2018),这凸显了人类受到交叉感染的可能性。病毒感染不断演变的威胁,以及最近 SARS-CoV-2 大流行对健康和社会经济造成的持久影响,都强调了对先进抗病毒药物筛选工具的迫切需要,这些工具可以快速实施,以加强对未来疫情爆发的准备和预防措施。在此,我们介绍了一种新型 RNA 荧光原位杂交(FISH)成像检测方法的开发和验证情况,该方法可作为 384 孔高通量快速反应平台用于抗病毒药物的发现。RNA-FISH 是一种功能强大的工具,可利用高含量成像平台观察培养细胞中的特定 mRNA。RNA-FISH 探针组的灵活性使其能够快速设计病毒基因组特异性探针,从而实现体外检测开发,以测试生物或小分子抑制剂对病毒复制的抑制作用。对 170 种抗病毒化合物进行的浓度反应筛选表明,RNA-FISH 检测和免疫荧光检测 (IFA) 对人类冠状病毒 HCoV-OC43 和 HCoV-229E 都有很强的相关性。此外,我们还成功地将该方法应用于 CCoV 1-71 株,证明了该方法的快速开发和部署,为评估抗病毒药物以应对未来潜在的新威胁开辟了新途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

SLAS Discovery
Chemistry-Analytical Chemistry
CiteScore
7.00
自引率
3.20%
发文量
58
审稿时长
39 days
期刊介绍:
Advancing Life Sciences R&D: SLAS Discovery reports how scientists develop and utilize novel technologies and/or approaches to provide and characterize chemical and biological tools to understand and treat human disease.
SLAS Discovery is a peer-reviewed journal that publishes scientific reports that enable and improve target validation, evaluate current drug discovery technologies, provide novel research tools, and incorporate research approaches that enhance depth of knowledge and drug discovery success.
SLAS Discovery emphasizes scientific and technical advances in target identification/validation (including chemical probes, RNA silencing, gene editing technologies); biomarker discovery; assay development; virtual, medium- or high-throughput screening (biochemical and biological, biophysical, phenotypic, toxicological, ADME); lead generation/optimization; chemical biology; and informatics (data analysis, image analysis, statistics, bio- and chemo-informatics). Review articles on target biology, new paradigms in drug discovery and advances in drug discovery technologies.
SLAS Discovery is of particular interest to those involved in analytical chemistry, applied microbiology, automation, biochemistry, bioengineering, biomedical optics, biotechnology, bioinformatics, cell biology, DNA science and technology, genetics, information technology, medicinal chemistry, molecular biology, natural products chemistry, organic chemistry, pharmacology, spectroscopy, and toxicology.
SLAS Discovery is a member of the Committee on Publication Ethics (COPE) and was published previously (1996-2016) as the Journal of Biomolecular Screening (JBS).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


