NEK2 promotes colorectal cancer progression by activating the TGF-β/Smad2 signaling pathway
IF 5
2区 医学
Q2 Medicine
引用次数: 0
Abstract
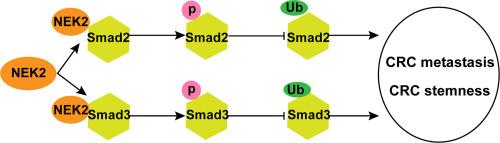
Colorectal cancer (CRC) is a prevalent malignancy with poor patient survival, and NIMA-associated kinase 2 (NEK2) has been implicated in the pathogenesis and progression of various cancers, including CRC. This study aimed to investigate the impact of NEK2 on CRC cell functionality and its interaction with the TGF-β/Smad signaling pathway. NEK2 expression in CRC tissues and cell lines was assessed, and its association with patient survival was analyzed. Functional assays, including NEK2 knockdown via lentiviral infection, RT-qPCR, Western blotting, CCK-8 assay, Transwell migration, invasion assays, and goblet cell formation assays, were employed to evaluate NEK2′s effects on CRC cell proliferation, migration, invasion, and stemness. Mechanistic studies explored the TGF-β/Smad2 signaling pathway, utilizing co-immunoprecipitation (Co-IP) and protein interaction analyses. In vivo experiments further evaluated NEK2′s role in tumor initiation, metastasis, and chemoresistance. NEK2 was found to be upregulated in CRC tissues and correlated with poor survival. NEK2 knockdown inhibited CRC cell behaviors, while NEK2 activated the TGF-β/Smad2 signaling pathway through Smad2/3 phosphorylation. Overexpression of Smad2/3 reversed NEK2 knockdown effects, confirming the importance of this pathway in CRC. In vivo, NEK2 promoted tumor initiation, metastasis, and chemoresistance, effects partially reversed by Smad2/3 overexpression. These findings reveal the critical role of NEK2 in CRC progression and underscore its potential as a therapeutic target, offering new insights into the molecular mechanisms driving CRC and informing targeted therapy development.
NEK2 通过激活 TGF-β/Smad2 信号通路促进结直肠癌进展
结肠直肠癌(CRC)是一种发病率高、患者生存率低的恶性肿瘤,而NIMA相关激酶2(NEK2)与包括CRC在内的多种癌症的发病机制和进展有关。本研究旨在探讨NEK2对CRC细胞功能的影响及其与TGF-β/Smad信号通路的相互作用。研究评估了 NEK2 在 CRC 组织和细胞系中的表达,并分析了其与患者生存的关系。通过慢病毒感染敲除 NEK2、RT-qPCR、Western 印迹、CCK-8 试验、Transwell 迁移、侵袭试验和上睑细胞形成试验等功能试验,评估了 NEK2 对 CRC 细胞增殖、迁移、侵袭和干性的影响。机理研究利用共免疫沉淀(Co-IP)和蛋白质相互作用分析探索了 TGF-β/Smad2 信号通路。体内实验进一步评估了 NEK2 在肿瘤发生、转移和化疗抵抗中的作用。研究发现,NEK2在CRC组织中上调,并与生存率低相关。敲除 NEK2 可抑制 CRC 细胞的行为,而 NEK2 可通过 Smad2/3 磷酸化激活 TGF-β/Smad2 信号通路。Smad2/3的过表达逆转了NEK2的敲除效应,证实了该通路在CRC中的重要性。在体内,NEK2促进了肿瘤的发生、转移和化疗抵抗,而Smad2/3的过表达可部分逆转这种效应。这些发现揭示了 NEK2 在 CRC 进展过程中的关键作用,并强调了其作为治疗靶点的潜力,为研究驱动 CRC 的分子机制提供了新的视角,并为靶向疗法的开发提供了信息。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Translational Oncology
ONCOLOGY-
CiteScore
8.40
自引率
2.00%
发文量
314
审稿时长
54 days
期刊介绍:
Translational Oncology publishes the results of novel research investigations which bridge the laboratory and clinical settings including risk assessment, cellular and molecular characterization, prevention, detection, diagnosis and treatment of human cancers with the overall goal of improving the clinical care of oncology patients. Translational Oncology will publish laboratory studies of novel therapeutic interventions as well as clinical trials which evaluate new treatment paradigms for cancer. Peer reviewed manuscript types include Original Reports, Reviews and Editorials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


