NNN type pincer Pd (II) complexes of pyridine-2,6-dicarboxamides: Catalytic activity and supramolecular formation
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
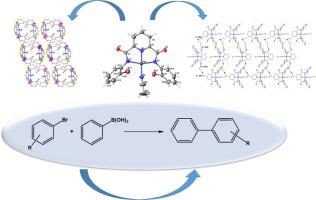
Pyridine-2,6-dicarboxamide, NNN pincer type pro-ligands, and their palladium complexes were synthesized. The prepared pro-ligands and complexes were characterized with several characterization techniques (FT-IR, 1H NMR, 13C NMR, and UV–vis analyses). The molecular structure of the acetonitrile-N2,N6-bis(2-bromophenyl)pyridine-2,6-dicarboxamidopalladium(II) complex has been determined by single crystal XRD measurement. This complex contains intramolecular and intermolecular H-bonds, C![]() H⋅⋅⋅π and face-to-face π⋅⋅⋅π interactions. In addition, two synthons were found in the crystal lattice that have been generated through hydrogen-bond interactions. Homo-synthons (), which forms through C
H⋅⋅⋅π and face-to-face π⋅⋅⋅π interactions. In addition, two synthons were found in the crystal lattice that have been generated through hydrogen-bond interactions. Homo-synthons (), which forms through C![]() H⋅⋅⋅O intermolecular interactions (2.816 Å for C15
H⋅⋅⋅O intermolecular interactions (2.816 Å for C15![]() H15⋅⋅⋅O1 and 2.603 Å for C13
H15⋅⋅⋅O1 and 2.603 Å for C13![]() H13⋅⋅⋅O2) and hetero-synthons (), that generated via C
H13⋅⋅⋅O2) and hetero-synthons (), that generated via C![]() H⋅⋅⋅N intermolecular interactions (C21
H⋅⋅⋅N intermolecular interactions (C21![]() H21C⋅⋅⋅N4, 3.439 Å). Furhermore, homo-synthons ( and ) through intermolecular contacts were determined between the palladium complex and the acetonitrile molecules. Furthermore, a quantitative analysis of the noncovalent interactions within the structure was performed using Hirshfeld surface and two-dimensional fingerprint plot analyses. The three-dimensional arrangement of the crystal packing was assessed during the energy-framework calculations, revealing that the electrostatic energy framework had a considerable influence on the dispersion energy framework. The [PdL5] complex provided a conversion of more than 98 % and a yield of more than 98 % in the shortest time among other catalysts, in which the Suzuki–Miyaura C
H21C⋅⋅⋅N4, 3.439 Å). Furhermore, homo-synthons ( and ) through intermolecular contacts were determined between the palladium complex and the acetonitrile molecules. Furthermore, a quantitative analysis of the noncovalent interactions within the structure was performed using Hirshfeld surface and two-dimensional fingerprint plot analyses. The three-dimensional arrangement of the crystal packing was assessed during the energy-framework calculations, revealing that the electrostatic energy framework had a considerable influence on the dispersion energy framework. The [PdL5] complex provided a conversion of more than 98 % and a yield of more than 98 % in the shortest time among other catalysts, in which the Suzuki–Miyaura C![]() C cross-coupling reaction (SMR) was carried out with the 4-bromotoluene substrate. The activity of [PdL5] complex was then examined in the reactions of two other substrates, 1-bromo-4-isobutylbenzene and 2-bromo-6-methoxynaphthalene. Although a yield of over 97 % and a conversion of over 97 % were observed in the reaction of 1-bromo-4-isobutylbenzene after two hours, the reaction of 2-bromo-6-methoxynaphthalene achieved complete conversion and a yield of over 99 % within just one hour.
C cross-coupling reaction (SMR) was carried out with the 4-bromotoluene substrate. The activity of [PdL5] complex was then examined in the reactions of two other substrates, 1-bromo-4-isobutylbenzene and 2-bromo-6-methoxynaphthalene. Although a yield of over 97 % and a conversion of over 97 % were observed in the reaction of 1-bromo-4-isobutylbenzene after two hours, the reaction of 2-bromo-6-methoxynaphthalene achieved complete conversion and a yield of over 99 % within just one hour.
吡啶-2,6-二羧酰胺的 NNN 型钳形钯 (II) 配合物:催化活性和超分子形成
合成了吡啶-2,6-二甲酰胺、NNN钳型原配体及其钯配合物。利用多种表征技术(傅立叶变换红外光谱、1H NMR、13C NMR 和紫外-可见光分析)对所制备的原配体和配合物进行了表征。通过单晶 XRD 测量确定了乙腈-N2,N6-双(2-溴苯基)吡啶-2,6-二羧酰胺钯(II)配合物的分子结构。该复合物含有分子内和分子间的 H 键、CH⋅⋅π 和面对面的 π⋅⋅π 相互作用。此外,在晶格中还发现了两个通过氢键相互作用产生的合成子。同合成子(R22(12))是通过 CH⋅⋅O 分子间相互作用形成的(C15H15⋅⋅O1 为 2.816 Å,C13H13⋅⋅O2 为 2.603 Å),异合成子(R21(8))是通过 CH⋅⋅N 分子间相互作用形成的(C21H21⋅C⋅⋅N4,3.439 Å)。此外,通过钯络合物与乙腈分子之间的分子间接触,还确定了同源合成物(R33(11) 和 R22(9))。此外,还利用 Hirshfeld 表面和二维指纹图谱分析法对结构内的非共价相互作用进行了定量分析。在能量框架计算过程中对晶体堆积的三维排列进行了评估,结果表明静电能量框架对分散能量框架有相当大的影响。在以 4-溴甲苯为底物进行的铃木-宫浦 CC 交叉偶联反应(SMR)中,[PdL5] 复合物在其他催化剂中以最短的时间实现了 98% 以上的转化率和 98% 以上的产率。随后,[PdL5] 复合物的活性又在另外两种底物--1-溴-4-异丁基苯和 2-溴-6-甲氧基萘的反应中得到了检验。虽然在 1-溴-4-异丁基苯的反应中,两个小时后就观察到了超过 97% 的产率和超过 97% 的转化率,但在 2-溴-6-甲氧基萘的反应中,仅一个小时就实现了完全转化和超过 99% 的产率。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


