A green chemistry approach for one-pot synthesis and antibacterial studies of 2,3′-bi-indole derivatives using Brønsted acid ionic liquid as an organocatalyst
IF 5.3
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
A cost-effective and environmentally friendly one-pot method for synthesizing 2,3′-bi-indole derivatives has been developed, utilizing a Brønsted acidic ionic liquid as an organocatalyst via tandem cyclization reaction. This process involves imine formation, nucleophilic addition, and cyclization, with water as the only by-product. The advantages of this protocol include metal-free, mild reaction conditions, easy workup, simple purification technique, broad substrate scope, high product yields, and lower E-factors. Furthermore, the synthesized products exhibit promising biological activities, and the antibacterial activities have been studied for some randomly taken synthesized compounds.
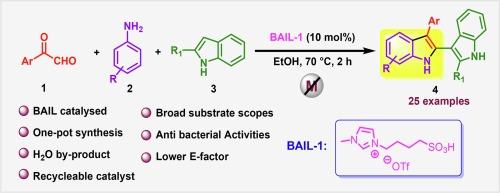
利用布氏酸离子液体作为有机催化剂,一锅合成 2,3′-双吲哚衍生物并进行抗菌研究的绿色化学方法
利用布氏酸性离子液体作为有机催化剂,通过串联环化反应合成 2,3′-双吲哚衍生物。这一过程包括亚胺形成、亲核加成和环化,唯一的副产物是水。该方案的优点包括无金属、反应条件温和、易于操作、纯化技术简单、底物范围广、产品收率高和 E 因子较低。此外,合成的产物具有良好的生物活性,并对随机合成的一些化合物进行了抗菌活性研究。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Liquids
化学-物理:原子、分子和化学物理
CiteScore
10.30
自引率
16.70%
发文量
2597
审稿时长
78 days
期刊介绍:
The journal includes papers in the following areas:
– Simple organic liquids and mixtures
– Ionic liquids
– Surfactant solutions (including micelles and vesicles) and liquid interfaces
– Colloidal solutions and nanoparticles
– Thermotropic and lyotropic liquid crystals
– Ferrofluids
– Water, aqueous solutions and other hydrogen-bonded liquids
– Lubricants, polymer solutions and melts
– Molten metals and salts
– Phase transitions and critical phenomena in liquids and confined fluids
– Self assembly in complex liquids.– Biomolecules in solution
The emphasis is on the molecular (or microscopic) understanding of particular liquids or liquid systems, especially concerning structure, dynamics and intermolecular forces. The experimental techniques used may include:
– Conventional spectroscopy (mid-IR and far-IR, Raman, NMR, etc.)
– Non-linear optics and time resolved spectroscopy (psec, fsec, asec, ISRS, etc.)
– Light scattering (Rayleigh, Brillouin, PCS, etc.)
– Dielectric relaxation
– X-ray and neutron scattering and diffraction.
Experimental studies, computer simulations (MD or MC) and analytical theory will be considered for publication; papers just reporting experimental results that do not contribute to the understanding of the fundamentals of molecular and ionic liquids will not be accepted. Only papers of a non-routine nature and advancing the field will be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


