A Regioselective, One-Pot, Transition-Metal-Free α-Alkylation of Quinone Monoacetals for Various Organic Transformations.
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Regioselective reactions of biologically significant quinones are challenging. An unprecedented advancement in quinone monoacetal (QMA) chemistry is proposed for constructing regioselective and less explored α-alkylated QMAs through the Morita-Baylis-Hillman (MBH) reaction. Electrophilic QMAs were transformed to nucleophilic umpolung reagents for aldol-type condensation with several electrophiles. Mechanistic studies reveal that solvent (TFE:water (1:1)) and in situ-generated potassium acetate accelerate the reaction. The ensuing MBH adducts were scalable and underwent several post-synthetic transformations and late-stage functionalization.
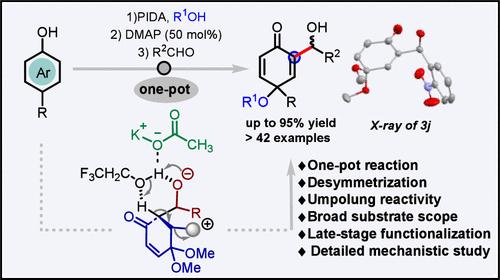
用于各种有机转化的醌单乙酸酯的区域选择性、一锅式、无过渡金属 α-烷基化。
具有生物学意义的醌类化合物的区域选择性反应具有挑战性。通过 Morita-Baylis-Hillman (MBH) 反应构建具有区域选择性且探索较少的α-烷基化 QMA,这是醌单缩醛(QMA)化学领域前所未有的进步。亲电的 QMA 被转化为亲核的umpolung 试剂,与多种亲电物发生醛醇缩合反应。机理研究表明,溶剂(TFE:水(1:1))和原位生成的醋酸钾会加速反应。随后生成的 MBH 加合物具有可扩展性,并经过了多次合成后转化和后期官能化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


