Stereodivergent Synthesis of 6,12-Guaianolide C1 Epimers via a Rationally Designed Oxy-Cope/Ene Reaction Cascade
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Nature synthesizes epimeric C1 guaianolide congeners, key components of major natural product classes, through a single structurally flexible macrocyclic germacranolide core. Our rationally designed elemanolide-type scaffold (5) now mimics this natural process, enabling the stereodivergent synthesis of both C1 epimers of 6,12-guaianolide lactone motifs. An oxy-Cope/ene cascade acts as the key step of this process, generating two distinct conformers of an intermediate germacranolide, each leading to a specific C1 epimer. Highly stereoselective redox manipulations follow, culminating in the efficient syntheses of diverse osmitopsin-type guaianolides.
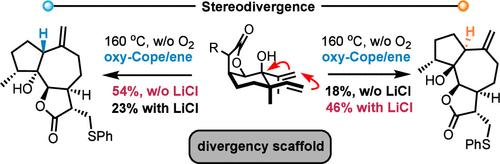
通过合理设计的 Oxy-Cope/Ene 反应级联立体异构合成 6,12-胍内酯 C1 表聚物
自然界通过一个结构灵活的大环germacranolide核心合成主要天然产品类别的关键成分--C1愈创木酚内酯的外延物。现在,我们合理设计的榄香内酯类支架(5)模拟了这一自然过程,能够立体异构合成 6,12- 愈创木酚内酯基团的两种 C1 表聚物。氧-科佩/烯级联反应是这一过程的关键步骤,它生成了中间体germacranolide的两种不同构象,每种构象都导致了特定的C1外延物。随后进行高度立体选择性的氧化还原操作,最终高效合成了多种渗透蛋白型愈创木酚内酯。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


