Synthesis and catalytic application to form cyclic carbonates of novel Pd(II) Cu(II), and Fe(II) benzoate-based Schiff base metal complexes
IF 2.1
3区 化学
Q3 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
This work describes the synthesis and characterisation of six novel Schiff base complexes, Cu(II), Pd(II), and Fe(II), which, under the right circumstances, function as very efficient catalysts for the production of cyclic carbonates from CO2 and epoxides. FT-IR spectroscopy, elemental analysis, UV–Vis spectroscopy, molar conductivity, melting point, and magnetic susceptibility measurements are among the spectroscopic methods used to characterize newly synthesized complexes. The molar conductivity values, ranging from 2.58 to 4.03 µS/cm, suggest that the complexes exhibit no molar conductivity. Co-catalysts, such as 4-(dimethylamino)pyridine, pyridine, triethylamine, and triphenyl phosphine, were used in these processes, both with and without one. 4-(Dimethylamino)pyridine was used as the co-catalyst in the catalytic studies. Additionally, a number of variables that affect the cycloaddition process were examined, including the temperature, CO2 pressure, reaction time, and co-catalyst. All novel catalysts exhibited exceptional catalytic activity and selectivity in the catalytic assays. Regarding the coupling of CO2 and epichlorohydrin as epoxides, the L2-Cu catalyst outperformed other catalysts in terms of catalytic activity (90.7%) and selectivity (98.9%).
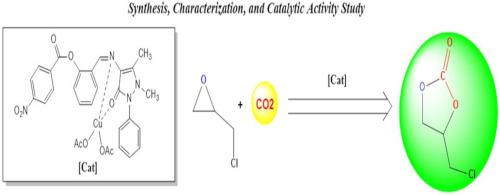
新型钯(II)、铜(II)和铁(II)苯甲酸基席夫碱金属配合物环状碳酸盐的合成和催化应用
本研究介绍了六种新型希夫碱配合物(Cu(II)、Pd(II) 和 Fe(II))的合成和表征,在适当的条件下,这些配合物可作为非常高效的催化剂,从二氧化碳和环氧化物中生产环状碳酸盐。傅立叶变换红外光谱法、元素分析法、紫外-可见光谱法、摩尔电导率、熔点和磁感应强度测量法等光谱学方法被用于表征新合成的复合物。摩尔电导率值在 2.58 至 4.03 µS/cm 之间,表明这些配合物不具有摩尔电导率。在这些过程中,使用了 4-(二甲基氨基)吡啶、吡啶、三乙胺和三苯基膦等辅助催化剂,其中既有使用辅助催化剂的,也有不使用辅助催化剂的。在催化研究中,4-(二甲基氨基)吡啶被用作辅助催化剂。此外,还研究了影响环化过程的一些变量,包括温度、二氧化碳压力、反应时间和助催化剂。在催化试验中,所有新型催化剂都表现出卓越的催化活性和选择性。在将 CO2 和环氧氯丙烷偶联为环氧化物方面,L2-铜催化剂的催化活性(90.7%)和选择性(98.9%)均优于其他催化剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organometallic Chemistry
化学-无机化学与核化学
CiteScore
4.40
自引率
8.70%
发文量
221
审稿时长
36 days
期刊介绍:
The Journal of Organometallic Chemistry targets original papers dealing with theoretical aspects, structural chemistry, synthesis, physical and chemical properties (including reaction mechanisms), and practical applications of organometallic compounds.
Organometallic compounds are defined as compounds that contain metal - carbon bonds. The term metal includes all alkali and alkaline earth metals, all transition metals and the lanthanides and actinides in the Periodic Table. Metalloids including the elements in Group 13 and the heavier members of the Groups 14 - 16 are also included. The term chemistry includes syntheses, characterizations and reaction chemistry of all such compounds. Research reports based on use of organometallic complexes in bioorganometallic chemistry, medicine, material sciences, homogeneous catalysis and energy conversion are also welcome.
The scope of the journal has been enlarged to encompass important research on organometallic complexes in bioorganometallic chemistry and material sciences, and of heavier main group elements in organometallic chemistry. The journal also publishes review articles, short communications and notes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


