A novel carbon quantum dot (CQD) synthesis method with cost-effective reactants and a definitive indication: Hot bubble synthesis (HBBBS)
IF 6.7
3区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
Journal of Science: Advanced Materials and Devices
Pub Date : 2024-10-24
DOI:10.1016/j.jsamd.2024.100797
引用次数: 0
Abstract
Carbon quantum dots (CQDs) are carbon-based biocompatible quantum dots that have low toxicity, are more soluble in water, have broad application areas, and the surface modification of these can be performed easily. In this study, we present a new CQD synthesis method with cost-effective reactants that can be easily found in laboratories are used. Besides, there is a definitive indication (bubble) for CQD production, unlike the other methods in the literature. The purification method of the CQDs was also optimized in this study. In the beginning of the synthesis, 3 times centrifugation (x3 CF) of the mixture was performed and the optimization of the purification method indicated that x3 CF before 3 days of dialysis membrane tubing (x3 CF & 3 DM) resulted in the formation of the purest CQDs. The characterization of the CQDs was done utilizing UV–VIS spectrophotometer, Fourier Transfer Infrared Spectroscopy (FT-IR), Zetasizer, fluorescence microscopy, Dynamic Light Scattering (DLS), Scanning Electron Microscope (SEM), Energy Dispersive Spectroscopy (EDS), and Transmission Electron Microscopy (TEM). It was concluded that the CQD formation depended on the mixing of sulphuric acid:acetone (2:1) ratio in a hot (140–165 °C) and an inert environment, and bubble coverage of the mixture surface (30–60 s). The bubble formation due to the reaction between sulphuric acid and pure acetone at high temperatures gives the developed method’s name of “Hot Bubble Synthesis” (HBBBS).
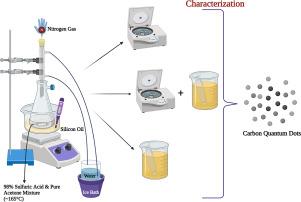
一种新型的碳量子点 (CQD) 合成方法,其反应物具有成本效益,并有明确的指示:热气泡合成法(HBBBS)
碳量子点(CQDs)是一种碳基生物相容性量子点,具有毒性低、水溶性强、应用领域广泛等特点,而且其表面修饰也很容易进行。在本研究中,我们提出了一种新的 CQD 合成方法,并使用了实验室中很容易找到的高性价比反应物。此外,与文献中的其他方法不同,该方法有明确的 CQD 生成指示(气泡)。本研究还优化了 CQD 的纯化方法。在合成初期,对混合物进行了 3 次离心(x3 CF),纯化方法的优化结果表明,在透析膜管(x3 CF & 3 DM)透析 3 天前进行 x3 CF 可形成最纯净的 CQD。利用紫外可见分光光度计(UV-VIS)、傅立叶红外光谱仪(FT-IR)、Zetasizer、荧光显微镜、动态光散射(DLS)、扫描电子显微镜(SEM)、能量色散光谱仪(EDS)和透射电子显微镜(TEM)对 CQDs 进行了表征。结果表明,CQD 的形成取决于硫酸与丙酮(2:1)在高温(140-165 °C)和惰性环境下的混合以及混合物表面的气泡覆盖(30-60 秒)。由于硫酸和纯丙酮在高温下发生反应而形成气泡,因此所开发的方法被命名为 "热气泡合成法"(HBBBS)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Science: Advanced Materials and Devices
Materials Science-Electronic, Optical and Magnetic Materials
CiteScore
11.90
自引率
2.50%
发文量
88
审稿时长
47 days
期刊介绍:
In 1985, the Journal of Science was founded as a platform for publishing national and international research papers across various disciplines, including natural sciences, technology, social sciences, and humanities. Over the years, the journal has experienced remarkable growth in terms of quality, size, and scope. Today, it encompasses a diverse range of publications dedicated to academic research.
Considering the rapid expansion of materials science, we are pleased to introduce the Journal of Science: Advanced Materials and Devices. This new addition to our journal series offers researchers an exciting opportunity to publish their work on all aspects of materials science and technology within the esteemed Journal of Science.
With this development, we aim to revolutionize the way research in materials science is expressed and organized, further strengthening our commitment to promoting outstanding research across various scientific and technological fields.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


