TOMM40 regulates hepatocellular and plasma lipid metabolism via an LXR-dependent pathway
IF 7
2区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
Objective
The gene encoding TOMM40 (Transporter of Outer Mitochondrial Membrane 40) is adjacent to that encoding APOE, which has a central role in lipid and lipoprotein metabolism. While human genetic variants near APOE and TOMM40 have been shown to be strongly associated with plasma lipid levels, a specific role for TOMM40 in lipid metabolism has not been established, and the present study was aimed at assessing this possibility.
Methods
TOMM40 was knocked down by siRNA in human hepatoma HepG2 cells, and effects on mitochondrial function, lipid phenotypes, and crosstalk between mitochondria, ER, and lipid droplets were examined. Additionally, hepatic and plasma lipid levels were measured in mice following shRNA-induced knockdown of Tomm40 shRNA.
Results
In HepG2 cells, TOMM40 knockdown upregulated expression of APOE and LDLR in part via activation of LXRB (NR1H2) by oxysterols, with consequent increased uptake of VLDL and LDL. This is in part due to disruption of mitochondria-endoplasmic reticulum contact sites, with resulting accrual of reactive oxygen species and non-enzymatically derived oxysterols. With TOMM40 knockdown, cellular triglyceride and lipid droplet content were increased, effects attributable in part to receptor-mediated VLDL uptake, since lipid staining was significantly reduced by concomitant suppression of either LDLR or APOE. In contrast, cellular cholesterol content was reduced due to LXRB-mediated upregulation of the ABCA1 transporter as well as increased production and secretion of oxysterol-derived cholic acid. Consistent with the findings in hepatoma cells, in vivo knockdown of TOMM40 in mice resulted in significant reductions of plasma triglyceride and cholesterol concentrations, reduced hepatic cholesterol and increased triglyceride content, and accumulation of lipid droplets leading to development of steatosis.
Conclusions
These findings demonstrate a role for TOMM40 in regulating hepatic lipid and plasma lipoprotein levels and identify mechanisms linking mitochondrial function with lipid metabolism.
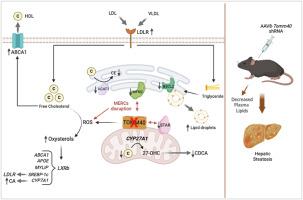
TOMM40 通过 LXR 依赖性途径调节肝细胞和血浆脂质代谢。
编码 TOMM40(线粒体外膜转运体 40)的基因与编码 APOE 的基因相邻,而 APOE 在脂质和脂蛋白代谢中起着核心作用。APOE 和 TOMM40 附近的人类基因变异与血浆脂质水平密切相关,但 TOMM40 在脂质代谢中的具体作用尚未确定。我们在此表明,抑制人肝癌细胞中的 TOMM40 会上调 APOE 和 LDLR 的表达,部分原因是氧杂醇激活了 LXRB (NR1H2),从而增加了对 VLDL 和 LDL 的吸收。这在一定程度上是由于线粒体-内质网接触点被破坏,导致活性氧和非酶促氧固醇的累积。敲除 TOMM40 后,细胞甘油三酯和脂滴含量增加,这种效应部分归因于受体介导的 VLDL 吸收,因为同时抑制 LDLR 或 APOE 会显著减少脂质染色。相反,由于 LXRB 介导的 ABCA1 转运体上调以及源于氧固醇的胆酸的产生和分泌增加,细胞胆固醇含量降低。与肝癌细胞中的研究结果一致,在小鼠体内敲除 TOMM40 会导致血浆甘油三酯和胆固醇浓度显著降低、肝脏胆固醇减少和甘油三酯含量增加,以及导致脂肪变性的脂滴积累。这些发现证明了 TOMM40 在调节肝脏脂质和血浆脂蛋白水平中的作用,并确定了线粒体功能与脂质代谢的联系机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
14.50
自引率
2.50%
发文量
219
审稿时长
43 days
期刊介绍:
Molecular Metabolism is a leading journal dedicated to sharing groundbreaking discoveries in the field of energy homeostasis and the underlying factors of metabolic disorders. These disorders include obesity, diabetes, cardiovascular disease, and cancer. Our journal focuses on publishing research driven by hypotheses and conducted to the highest standards, aiming to provide a mechanistic understanding of energy homeostasis-related behavior, physiology, and dysfunction.
We promote interdisciplinary science, covering a broad range of approaches from molecules to humans throughout the lifespan. Our goal is to contribute to transformative research in metabolism, which has the potential to revolutionize the field. By enabling progress in the prognosis, prevention, and ultimately the cure of metabolic disorders and their long-term complications, our journal seeks to better the future of health and well-being.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


