Recent pharmacological insights on abating toxic protein species burden in neurological disorders: Emphasis on 26S proteasome activation
IF 5.2
2区 医学
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
Abstract
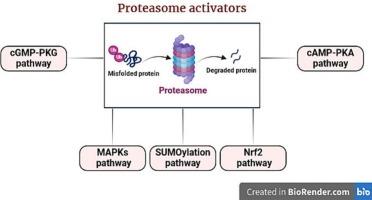
Protein homeostasis (proteostasis) refers to the plethora of mechanisms that safeguard the proper folding of the newly synthesized proteins. It entails various intricately regulated cues that demolish the toxic protein species to prevent their aggregation. The ubiquitin-proteasome system (UPS) is recognized as a salient protein degradation system, with a substantial role in maintaining proteostasis. However, under certain circumstances the protein degradation capacity of the UPS is overwhelmed, leading to the accumulation of misfolded proteins. Several neurodegenerative disorders, such as Alzheimer's disease, Parkinson's disease, Huntington disease, and amyotrophic lateral sclerosis are characterized with the presence of protein aggregates and proteinopathy. Accordingly, enhancing the 26S proteasome degradation activity might delineate a pioneering approach in targeting various proteotoxic disorders. Regrettably, the exact molecular approaches that enhance the proteasomal activity are still not fully understood. Therefore, this review aimed to underscore several signaling cascades that might restore the degradation capacity of this molecular machine. In this review, we discuss the different molecular components of the UPS and how 26S proteasomes are deleteriously affected in many neurodegenerative diseases. Moreover, we summarize different signaling pathways that can be utilized to renovate the 26S proteasome functional capacity, alongside currently known druggable targets in this circuit and various classes of proteasome activators.
关于减轻神经系统疾病中有毒蛋白质负担的最新药理学见解:重点关注 26S 蛋白酶体的激活。
蛋白质稳态(proteostasis)是指保障新合成蛋白质正确折叠的大量机制。它包括各种错综复杂的调控线索,这些线索能清除有毒的蛋白质种类,防止其聚集。泛素-蛋白酶体系统(UPS)是公认的重要蛋白质降解系统,在维持蛋白质稳态方面发挥着重要作用。然而,在某些情况下,泛素-蛋白酶体系统的蛋白质降解能力会不堪重负,导致错误折叠蛋白质的积累。一些神经退行性疾病,如阿尔茨海默病、帕金森病、亨廷顿病和肌萎缩侧索硬化症,都以蛋白质聚集和蛋白质病变为特征。因此,增强 26S 蛋白酶体的降解活性可能是针对各种蛋白毒性疾病的一种开创性方法。遗憾的是,提高蛋白酶体活性的确切分子方法仍未完全明了。因此,本综述旨在强调几种可能恢复这一分子机器降解能力的信号级联。在这篇综述中,我们讨论了 UPS 的不同分子成分,以及 26S 蛋白酶体如何在许多神经退行性疾病中受到有害影响。此外,我们还总结了可用于修复 26S 蛋白酶体功能的不同信号通路,以及该通路中目前已知的可药物靶点和各类蛋白酶体激活剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Life sciences
医学-药学
CiteScore
12.20
自引率
1.60%
发文量
841
审稿时长
6 months
期刊介绍:
Life Sciences is an international journal publishing articles that emphasize the molecular, cellular, and functional basis of therapy. The journal emphasizes the understanding of mechanism that is relevant to all aspects of human disease and translation to patients. All articles are rigorously reviewed.
The Journal favors publication of full-length papers where modern scientific technologies are used to explain molecular, cellular and physiological mechanisms. Articles that merely report observations are rarely accepted. Recommendations from the Declaration of Helsinki or NIH guidelines for care and use of laboratory animals must be adhered to. Articles should be written at a level accessible to readers who are non-specialists in the topic of the article themselves, but who are interested in the research. The Journal welcomes reviews on topics of wide interest to investigators in the life sciences. We particularly encourage submission of brief, focused reviews containing high-quality artwork and require the use of mechanistic summary diagrams.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


