Exploring the molecular underpinnings of macrosomia in gestational diabetes mellitus: The role of EGFR signaling and placental syncytiotrophoblast
IF 5.2
2区 医学
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
Abstract
Macrosomia, which is frequently associated with gestational diabetes mellitus (GDM), is linked to maternal glycemic control during gestation. When GDM is complicated by macrosomia (GDMM), the placenta exhibits increased mass, underscoring its role as a critical nexus for maternal-fetal nutrient exchange. Despite this recognized correlation, the underlying mechanisms propelling placental hypertrophy have remained elusive. Our study leveraged single-cell RNA transcriptome sequencing of GDMM placental tissues to pinpoint the specific syncytiotrophoblast (SCT) subsets that regulate placental dimensions. Notably, we observed pronounced upregulation of the epidermal growth factor receptor (EGFR) and its corresponding ligands, with a particular emphasis on the autoregulatory cascade involving the glycoprotein hormone alpha subunit (CGA), EGFR, and the transcription factor GATA binding protein 2 (GATA2), as well as perturbations in hormonal homeostasis within the SCT. Furthermore, our cell interaction analysis revealed an enhanced interplay between myeloid cells and SCT3, augmenting the EGFR signaling pathway. These molecular exchanges underscore the pivotal role of the placental immune microenvironment in the etiology of macrosomia, shedding light on the pathophysiology of GDMM and paving the way for novel therapeutic approaches.
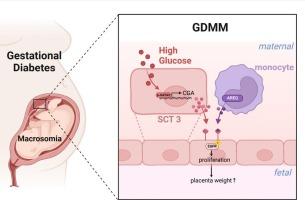
探索妊娠期糖尿病大畸形的分子基础:表皮生长因子受体信号转导和胎盘合体滋养细胞的作用。
妊娠期糖尿病(GDM)常伴有巨大胎儿症,这与母亲在妊娠期间的血糖控制有关。当妊娠期糖尿病并发巨大胎儿症(GDMM)时,胎盘会显示出增大的质量,突出了其作为母胎营养交换关键纽带的作用。尽管存在这种公认的相关性,但推动胎盘肥大的潜在机制仍然难以捉摸。我们的研究利用 GDMM 胎盘组织的单细胞 RNA 转录组测序来确定调节胎盘尺寸的特定合胞滋养细胞(SCT)亚群。值得注意的是,我们观察到表皮生长因子受体(EGFR)及其相应配体的明显上调,尤其是涉及糖蛋白激素α亚基(CGA)、EGFR和转录因子GATA结合蛋白2(GATA2)的自调节级联,以及SCT内激素平衡的紊乱。此外,我们的细胞相互作用分析表明,髓系细胞与 SCT3 之间的相互作用增强,从而加强了表皮生长因子受体的信号通路。这些分子交换强调了胎盘免疫微环境在巨型畸形病因学中的关键作用,揭示了GDMM的病理生理学,并为新的治疗方法铺平了道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Life sciences
医学-药学
CiteScore
12.20
自引率
1.60%
发文量
841
审稿时长
6 months
期刊介绍:
Life Sciences is an international journal publishing articles that emphasize the molecular, cellular, and functional basis of therapy. The journal emphasizes the understanding of mechanism that is relevant to all aspects of human disease and translation to patients. All articles are rigorously reviewed.
The Journal favors publication of full-length papers where modern scientific technologies are used to explain molecular, cellular and physiological mechanisms. Articles that merely report observations are rarely accepted. Recommendations from the Declaration of Helsinki or NIH guidelines for care and use of laboratory animals must be adhered to. Articles should be written at a level accessible to readers who are non-specialists in the topic of the article themselves, but who are interested in the research. The Journal welcomes reviews on topics of wide interest to investigators in the life sciences. We particularly encourage submission of brief, focused reviews containing high-quality artwork and require the use of mechanistic summary diagrams.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


