Defective macrophage efferocytosis in advanced atherosclerotic plaque and mitochondrial therapy
IF 5.2
2区 医学
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
Abstract
Atherosclerosis (AS) is a chronic inflammatory disease primarily affecting large and medium-sized arterial vessels, characterized by lipoprotein disorders, intimal thickening, smooth muscle cell proliferation, and the formation of vulnerable plaques. Macrophages (MΦs) play a vital role in the inflammatory response throughout all stages of atherosclerotic development and are considered significant therapeutic targets. In early lesions, macrophage efferocytosis rapidly eliminates harmful cells. However, impaired efferocytosis in advanced plaques perpetuates the inflammatory microenvironment of AS. Defective efferocytosis has emerged as a key factor in atherosclerotic pathogenesis and the progression to severe cardiovascular disease. Herein, this review probes into investigate the potential mechanisms at the cellular, molecular, and organelle levels underlying defective macrophage efferocytosis in advanced lesion plaques. In the inflammatory microenvironments of AS with interactions among diverse inflammatory immune cells, impaired macrophage efferocytosis is strongly linked to multiple factors, such as a lower absolute number of phagocytes, the aberrant expression of crucial molecules, and impaired mitochondrial energy provision in phagocytes. Thus, focusing on molecular targets to enhance macrophage efferocytosis or targeting mitochondrial therapy to restore macrophage metabolism homeostasis has emerged as a potential strategy to mitigate the progression of advanced atherosclerotic plaque, providing various treatment options.
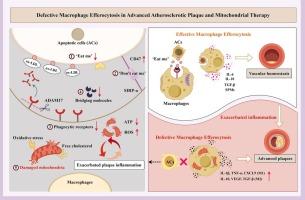
晚期动脉粥样硬化斑块中巨噬细胞排泄功能缺陷与线粒体疗法。
动脉粥样硬化(AS)是一种慢性炎症性疾病,主要影响大、中型动脉血管,其特征是脂蛋白紊乱、内膜增厚、平滑肌细胞增生和易损斑块的形成。巨噬细胞(MΦ)在动脉粥样硬化发展的各个阶段的炎症反应中都扮演着重要角色,被认为是重要的治疗靶点。在早期病变中,巨噬细胞的吞噬作用可迅速清除有害细胞。然而,晚期斑块中的脱落细胞功能受损会使强直性脊柱炎的炎症微环境持续存在。排出细胞功能缺陷已成为动脉粥样硬化发病机制和严重心血管疾病进展的关键因素。本综述将探讨晚期病变斑块中巨噬细胞渗出缺陷的细胞、分子和细胞器水平的潜在机制。在强直性脊柱炎的炎性微环境中,各种炎性免疫细胞之间相互作用,巨噬细胞吞噬功能受损与多种因素密切相关,如吞噬细胞绝对数量减少、关键分子表达异常、吞噬细胞线粒体能量供应受损等。因此,关注分子靶点以增强巨噬细胞的吞噬功能,或靶向线粒体治疗以恢复巨噬细胞代谢平衡,已成为缓解晚期动脉粥样硬化斑块进展的潜在策略,提供了多种治疗方案。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Life sciences
医学-药学
CiteScore
12.20
自引率
1.60%
发文量
841
审稿时长
6 months
期刊介绍:
Life Sciences is an international journal publishing articles that emphasize the molecular, cellular, and functional basis of therapy. The journal emphasizes the understanding of mechanism that is relevant to all aspects of human disease and translation to patients. All articles are rigorously reviewed.
The Journal favors publication of full-length papers where modern scientific technologies are used to explain molecular, cellular and physiological mechanisms. Articles that merely report observations are rarely accepted. Recommendations from the Declaration of Helsinki or NIH guidelines for care and use of laboratory animals must be adhered to. Articles should be written at a level accessible to readers who are non-specialists in the topic of the article themselves, but who are interested in the research. The Journal welcomes reviews on topics of wide interest to investigators in the life sciences. We particularly encourage submission of brief, focused reviews containing high-quality artwork and require the use of mechanistic summary diagrams.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


