Are we better together? Addressing a combined treatment of pitavastatin and temozolomide for brain cancer
IF 4.2
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Pitavastatin is commonly prescribed to treat hypercholesterolemia through the regulation of cholesterol biosynthesis. Interestingly, it has also demonstrated a great potential for treating brain tumors, although the detailed cytotoxic mechanism, particularly in glioblastoma, remains incompletely understood. This work explores the activity of pitavastatin in 2D and 3D glioblastoma models, in an attempt to provide a more representative and robust overview of its anticancer potential in glioblastoma. The results show that not only is pitavastatin 10-1000 times-fold more effective in reducing tumoral metabolic activity than temozolomide, but also demonstrate a synergistic activity with this alkylating drug. In addition, low micromolar concentrations of this statin strongly impair the growth and the invasion ability of multicellular tumor spheroids. The obtained qRT-PCR and proteomics data highlight the modulation of cell death via apoptosis (BAX/BCL2, CASP9) and autophagy (BECN1, BNIP3, BNIP3L and LC3B), as well as an epithelial to mesenchymal transition blockage (HTRA1, SERPINE1, WNT5A, ALDH3B1 and EPHA2) and remodeling of the extracellular matrix (VCAN, SERPINE1 and TGFBI). Overall, these results lay the foundation for further investigations on the potential combinatory clinical treatment with temozolomide.
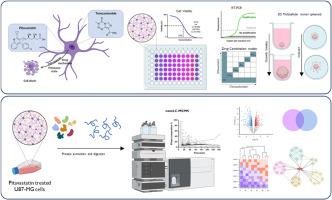
我们合作得更好吗?解决匹伐他汀和替莫唑胺联合治疗脑癌的问题。
匹伐他汀是通过调节胆固醇的生物合成来治疗高胆固醇血症的常用药物。有趣的是,它在治疗脑肿瘤方面也显示出巨大的潜力,尽管人们对其详细的细胞毒性机理,尤其是在胶质母细胞瘤中的机理仍不完全清楚。这项研究探讨了匹伐他汀在二维和三维胶质母细胞瘤模型中的活性,试图对其在胶质母细胞瘤中的抗癌潜力提供更具代表性和更有力的概述。结果表明,匹伐他汀在降低肿瘤代谢活性方面的效果不仅是替莫唑胺的10-1000倍,而且还显示出与这种烷化药物的协同活性。此外,低微摩尔浓度的他汀类药物会强烈影响多细胞肿瘤球体的生长和侵袭能力。获得的 qRT-PCR 和蛋白质组学数据强调了通过细胞凋亡(BAX/BCL2、CASP9)和自噬(BECN1、BNIP3、BNIP3L 和 LC3B)调节细胞死亡,以及阻止上皮细胞向间充质转化(HTRA1、SERPINE1、WNT5A、ALDH3B1 和 EPHA2)和重塑细胞外基质(VCAN、SERPINE1 和 TGFBI)。总之,这些结果为进一步研究替莫唑胺的潜在联合临床治疗奠定了基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.00
自引率
0.00%
发文量
572
审稿时长
34 days
期刊介绍:
The European Journal of Pharmacology publishes research papers covering all aspects of experimental pharmacology with focus on the mechanism of action of structurally identified compounds affecting biological systems.
The scope includes:
Behavioural pharmacology
Neuropharmacology and analgesia
Cardiovascular pharmacology
Pulmonary, gastrointestinal and urogenital pharmacology
Endocrine pharmacology
Immunopharmacology and inflammation
Molecular and cellular pharmacology
Regenerative pharmacology
Biologicals and biotherapeutics
Translational pharmacology
Nutriceutical pharmacology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


