Quantitative evaluation of adalimumab and anti-adalimumab antibodies in sera using a surface plasmon resonance biosensor
IF 2.5
3区 医学
Q2 MEDICAL LABORATORY TECHNOLOGY
引用次数: 0
Abstract
Objectives
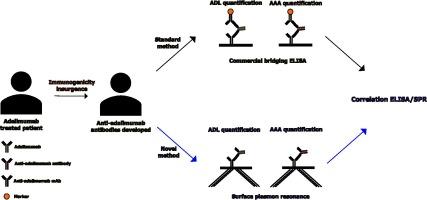
Monitoring of therapeutic antibody adalimumab (ADL) and of anti-adalimumab antibodies (AAA) in autoimmune diseases patients' sera has achieved increased attention since several studies showed a correlation between AAA levels and treatment failure. We evaluated a new surface plasmon resonance (SPR)-based method that, with slight changes in the analysis condition and in the ligand immobilized on the chip surface, allows to monitor both AAA and ADL. This new label-free method does not require sample pretreatments, and it is fully automated, only requiring the preparation of the chip, which can be used for multiple analysis, and the preparation of the sample sets.
Design & Methods
Sera from ADL-treated patients (n = 47) and controls (n = 13) were included in this study. Quantitative analysis of AAA and ADL were performed separately using a new SPR-biosensor, and a commercially available ELISA kit. Agreement was defined by overall, positive, and negative agreement. Wilson Score was used to calculate confidence intervals (CI) on binomial probability and Spearman’s rho and Bland-Altman test were used to assess correlations.
Results
ELISA and SPR-based assay were able to identify circulating AAA in ADL-treated patients, with the percentage of positivity varying among the methods, with an overall agreement of 79%. AAA were detected in 18 (38 %) out of the 47 treated patients by the ELISA whereas SPR-based assay detected 10 (21 %) out of 47 samples.
Conclusions
Real-time label free SPR-based protocol for both AAA and ADL quantification has been set-up. Although quantitative differences were observed when compared with ELISA, the agreement among methodologies was high, particularly for ADL quantification within the therapeutic window of the drug.
利用表面等离子体共振生物传感器定量评估血清中的阿达木单抗和抗阿达木单抗抗体
目的:自从几项研究表明阿达木单抗抗体水平与治疗失败之间存在相关性以来,对自身免疫性疾病患者血清中的治疗抗体阿达木单抗(ADL)和抗阿达木单抗抗体(AAA)进行监测就受到了越来越多的关注。我们评估了一种基于表面等离子体共振(SPR)的新方法,只要稍微改变分析条件和固定在芯片表面的配体,就能同时监测 AAA 和 ADL。这种新的无标记方法不需要对样品进行预处理,而且是全自动的,只需要准备芯片(可用于多次分析)和准备样品集:本研究纳入了ADL治疗患者(47人)和对照组(13人)的血清。使用新型 SPR 生物传感器和市售 ELISA 试剂盒分别对 AAA 和 ADL 进行定量分析。一致性由总体、阳性和阴性一致性定义。结果:ELISA和基于SPR的检测方法能够识别ADL治疗患者体内的循环AAA,不同方法的阳性率不同,总体一致性为79%。ELISA法检测出47名接受治疗的患者中有18人(38%)体内存在AAA,而SPR法检测出47份样本中有10人(21%)体内存在AAA:结论:基于实时无标记 SPR 法的 AAA 和 ADL 定量方案已经建立。尽管与酶联免疫吸附法相比在定量方面存在差异,但各种方法之间的一致性很高,特别是在药物治疗窗内的 ADL 定量方面。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Clinical biochemistry
医学-医学实验技术
CiteScore
5.10
自引率
0.00%
发文量
151
审稿时长
25 days
期刊介绍:
Clinical Biochemistry publishes articles relating to clinical chemistry, molecular biology and genetics, therapeutic drug monitoring and toxicology, laboratory immunology and laboratory medicine in general, with the focus on analytical and clinical investigation of laboratory tests in humans used for diagnosis, prognosis, treatment and therapy, and monitoring of disease.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


