Hepatocyte-specific SLC27A4 deletion ameliorates nonalcoholic fatty liver disease in mice via suppression of phosphatidylcholine-mediated PXR activation
IF 10.8
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
Background
The protein Solute carrier family 27 member 4 (SLC27A4) is crucial for fatty acid synthesis and β-oxidation, but its role in hepatic steatosis and nonalcoholic fatty liver disease (NAFLD) progression is not fully understood.
Methods
Mice with AAV-mediated overexpression of Slc27a4 in liver and hepatocytes-specific deletion of Slc27a4 were fed a standard chow diet, a high-fat diet (HFD), or a methionine and choline-deficient diet (MCD). Serum and liver tissues were collected and analyzed by biochemical assay, histology, lipidomic analysis, RNA-seq analysis, qPCR, western blot and immunofluorescence.
Results
This study found elevated expression of SLC27A4 in individuals with NAFLD and OAPA-treated MPHs cells, leading to increased lipid accumulation and diet-induced liver steatosis, inflammation, and fibrosis. Conversely, hepatocyte-specific deletion of Slc27a4 improved the development of both NAFLD and NASH. SLC27A4 overexpression resulted in increased hepatic pregnane X receptor (PXR) expression and accumulation of phosphatidylcholine (PC), which activates PXR signaling and inducing SLC27A4 expression. PXR overexpression hinders the protective impact of Slc27a4 deletion on lipid accumulation and inflammation, whereas its deficiency in mice reduces the effect of Slc27a4 overexpression on NAFLD development.
Conclusion
These results indicate that SLC27A4 plays a critical role of lipid accumulation and inflammation, and is implicated in the development of NAFLD progression, rendering it potentially actionable target for NAFLD treatment.
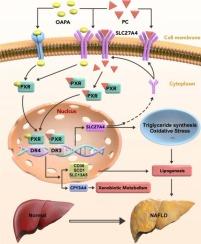
通过抑制磷脂酰胆碱介导的 PXR 激活,肝细胞特异性 SLC27A4 缺失可改善小鼠的非酒精性脂肪肝。
背景:蛋白质溶质运载家族27成员4(SLC27A4)对脂肪酸合成和β-氧化至关重要,但其在肝脂肪变性和非酒精性脂肪肝(NAFLD)进展中的作用尚未完全清楚:方法:用AAV介导在肝脏中过表达Slc27a4和肝细胞特异性缺失Slc27a4的小鼠,分别喂食标准饲料、高脂饲料(HFD)或蛋氨酸和胆碱缺乏饲料(MCD)。收集血清和肝组织,并通过生化测定、组织学、脂质组分析、RNA-seq分析、qPCR、Western印迹和免疫荧光等方法进行分析:结果:该研究发现,SLC27A4在非酒精性脂肪肝患者和经OAPA处理的MPHs细胞中表达升高,导致脂质蓄积增加,饮食诱发肝脏脂肪变性、炎症和纤维化。相反,肝细胞特异性缺失 Slc27a4 会改善非酒精性脂肪肝和非酒精性脂肪性肝炎的发展。SLC27A4过表达会导致肝脏孕烷X受体(PXR)表达和磷脂酰胆碱(PC)积累增加,从而激活PXR信号转导并诱导SLC27A4表达。PXR的过表达阻碍了Slc27a4缺失对脂质积累和炎症的保护作用,而PXR的缺乏则降低了Slc27a4过表达对非酒精性脂肪肝发展的影响:这些结果表明,SLC27A4 在脂质蓄积和炎症中起着关键作用,并与非酒精性脂肪肝的发展进程有关,因此是治疗非酒精性脂肪肝的潜在靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Metabolism: clinical and experimental
医学-内分泌学与代谢
CiteScore
18.90
自引率
3.10%
发文量
310
审稿时长
16 days
期刊介绍:
Metabolism upholds research excellence by disseminating high-quality original research, reviews, editorials, and commentaries covering all facets of human metabolism.
Consideration for publication in Metabolism extends to studies in humans, animal, and cellular models, with a particular emphasis on work demonstrating strong translational potential.
The journal addresses a range of topics, including:
- Energy Expenditure and Obesity
- Metabolic Syndrome, Prediabetes, and Diabetes
- Nutrition, Exercise, and the Environment
- Genetics and Genomics, Proteomics, and Metabolomics
- Carbohydrate, Lipid, and Protein Metabolism
- Endocrinology and Hypertension
- Mineral and Bone Metabolism
- Cardiovascular Diseases and Malignancies
- Inflammation in metabolism and immunometabolism
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


