Microglia phagocytosis of PNNs mediates PV-positive interneuron dysfunction and associated gamma oscillations in neuroinflammation-induced cognitive impairment in mice
IF 4.6
2区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
Neuroinflammation, characterized by activation of glial cells, plays a critical role in central nervous system disorders. However, the precise mechanisms of neuroinflammation contributing to cognitive impairment remain elusive. Perineuronal nets (PNNs) are extracellular matrixes that envelop the cell bodies and dendrites of parvalbumin (PV)-positive interneurons and may be mediated by apolipoprotein E (ApoE) gene. To investigate whether disruption of PNNs associated with ApoE is implicated in neuroinflammation-induced cognitive impairment, we established a neuroinflammation model by administering lipopolysaccharides (LPS) at 0.5 mg/kg for 7 consecutive days. Cognitive function was assessed using the open field, Y-maze, and novel object recognition tests, and neural oscillations were also recorded. Furthermore, differentially expressed genes in microglia within the hippocampus were identified through single-cell RNA sequencing analysis. Overexpression of hyaluronan and proteoglycan link protein 1 (Hapln1) and ApoE knockdown were carried out through adeno-associated virus (AAV) injection to C57BL/6J mice and CX3CR1-CreERT2 mice, respectively. It was found that LPS-induced neuroinflammation impaired cognitive function by reducing PNNs and PV-positive interneurons’ outputs, as well as disrupting gamma (γ) oscillations in the hippocampal CA1. Overexpression of Hapln1 was able to restore PV-positive interneurons and γ oscillations, ultimately alleviating the cognitive impairment. Mechanistically, LPS-triggered microglial activation leads to the phagocytosis of PNNs, a process influenced by ApoE. Notably, prevention of PNNs engulfment through targeting microglial ApoE in the CA1 improved cognitive impairment. Collectively, our study suggested that microglial phagocytosis of PNNs plays a key role in neuroinflammation-induced cognitive impairment, which is probably mediated by the ApoE.
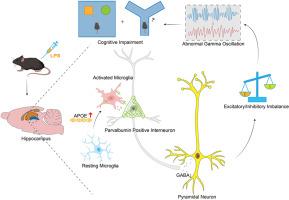
在神经炎症诱导的小鼠认知障碍中,小胶质细胞对 PNNs 的吞噬介导了 PV 阳性中间神经元功能障碍和相关的伽马振荡。
以神经胶质细胞活化为特征的神经炎症在中枢神经系统疾病中起着至关重要的作用。然而,导致认知障碍的神经炎症的确切机制仍然难以捉摸。神经元周围网(PNNs)是包裹副发光素(PV)阳性中间神经元细胞体和树突的细胞外基质,可能由载脂蛋白 E(ApoE)基因介导。为了研究与载脂蛋白 E 相关的 PNNs 的破坏是否与神经炎症引起的认知障碍有关,我们建立了一个神经炎症模型,通过连续 7 天以 0.5 毫克/千克的剂量给药脂多糖(LPS)。认知功能通过开阔地、Y-迷宫和新物体识别测试进行评估,神经振荡也被记录下来。此外,还通过单细胞RNA测序分析确定了海马内小胶质细胞中的差异表达基因。通过向C57BL/6J小鼠和CX3CR1-CreERT2小鼠注射腺相关病毒(AAV),分别实现了透明质酸和蛋白多糖连接蛋白1(Hapln1)的过表达和载脂蛋白E的敲除。研究发现,LPS诱导的神经炎症会减少PNNs和PV阳性中间神经元的输出,并破坏海马CA1的伽马(γ)振荡,从而损害认知功能。过量表达Hapln1能够恢复PV阳性中间神经元和γ振荡,最终缓解认知障碍。从机制上讲,LPS 触发的小胶质细胞活化导致了 PNNs 的吞噬,这一过程受到载脂蛋白 E 的影响。值得注意的是,通过靶向 CA1 中的小胶质细胞载脂蛋白E来阻止 PNNs 吞噬,可以改善认知障碍。总之,我们的研究表明,小胶质细胞吞噬 PNNs 在神经炎症诱导的认知障碍中起着关键作用,而这可能是由载脂蛋白E介导的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Neuropharmacology
医学-神经科学
CiteScore
10.00
自引率
4.30%
发文量
288
审稿时长
45 days
期刊介绍:
Neuropharmacology publishes high quality, original research and review articles within the discipline of neuroscience, especially articles with a neuropharmacological component. However, papers within any area of neuroscience will be considered. The journal does not usually accept clinical research, although preclinical neuropharmacological studies in humans may be considered. The journal only considers submissions in which the chemical structures and compositions of experimental agents are readily available in the literature or disclosed by the authors in the submitted manuscript. Only in exceptional circumstances will natural products be considered, and then only if the preparation is well defined by scientific means. Neuropharmacology publishes articles of any length (original research and reviews).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


