Zebrafish as a model organism to study sporadic Alzheimer's disease: Behavioural, biochemical and histological validation
IF 4.6
2区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
Alzheimer's disease (AD) is a global burden to the healthcare system with no viable treatment options till date. Rodents and primates have been extensively used as models for understanding AD pathogenesis and identifying therapeutic targets. However, the focus is now shifting towards developing alternate models. Zebrafish is emerging as a preferred model for neurodegenerative conditions because of its simple nervous system, highly conserved genome and short duration required to model disease condition. The present study is aimed to develop streptozotocin (STZ)-induced model of sporadic AD (sAD) in zebrafish. STZ was administered to adult zebrafish (4–6 mo) at different doses (1 to 50 mg/kg body weight, intracerebroventricularly). Kaplan-Meier survival analysis revealed time and dose dependent mortality in the zebrafish administered with STZ. Based on survival analysis, 1 to 10 mg/kg body weight of STZ was selected for behavioural, molecular and histological studies. STZ administered fish had anxiety-like and stress behaviour in novel tank and light/dark preference tests. STZ-induced cognitive and memory deficits assessed using novel object recognition and spatial alternation tests. Further, expression of markers of amyloidogenic pathway (appa and bace1) were increased in terms of mRNA and protein levels in a time and dose dependent manner following STZ administration. However, expression of non-amyloidogenic pathway mediator (adam10) was reduced at both mRNA and protein level. Histological assessment using hematoxylin and eosin, and Nissl stain revealed loss of neurons in STZ administered fish. The ratio of phosphor-tauser396/total-tau was increased in STZ administered fish. Based on these findings, 5 mg/kg body weight of STZ was found to be most appropriate dose to exhibit sAD phenotype. Mass spectrometric analysis confirmed the presence of amyloid beta oligomers in brains of STZ administered fish. Transmission electron microscopy also showed the presence of higher order insoluble amyloid fibrils with twists. Immunohistochemical analysis revealed amyloid beta deposits in brain of STZ administered fish. Golgi-cox staining indicated decreased number of dendrites, whereas microglia had increased density, span ratio, soma area and lacunarity. The results of the present study demonstrate presence of AD hallmarks and phenotype in zebrafish 7 days post STZ administration (5 mg/kg). The study validates the potential of STZ-induced sAD in zebrafish as a reliable model for studying pathophysiology and rapid screening of therapeutic molecules against sAD.
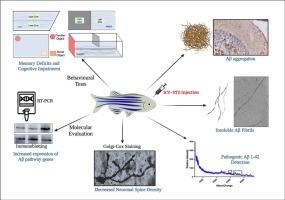
斑马鱼作为研究散发性阿尔茨海默病的模式生物:行为、生化和组织学验证。
阿尔茨海默病(AD)是全球医疗系统的一大负担,迄今为止还没有可行的治疗方案。啮齿类动物和灵长类动物被广泛用作了解阿尔茨海默病发病机制和确定治疗靶点的模型。然而,现在的重点正在转向开发替代模型。斑马鱼因其简单的神经系统、高度保守的基因组和较短的建模时间,正逐渐成为神经退行性疾病的首选模型。本研究旨在开发链脲佐菌素(STZ)诱导的斑马鱼散发性注意力缺失症(sAD)模型。研究人员给成年斑马鱼(4-6 个月)注射不同剂量的 STZ(1 至 50 毫克/千克体重,脑室内注射)。卡普兰-米尔存活率分析显示,使用 STZ 的斑马鱼死亡率与时间和剂量有关。根据存活率分析,选择每公斤体重 1 至 10 毫克的 STZ 进行行为、分子和组织学研究。使用 STZ 的斑马鱼在新鱼缸和光/暗偏好测试中表现出焦虑和应激样行为。使用新物体识别和空间交替测试评估了 STZ 诱导的认知和记忆缺陷。此外,STZ 施用后,淀粉样蛋白生成途径的标记物(appa 和 bace1)的 mRNA 和蛋白质水平的表达增加,且呈时间和剂量依赖性。然而,非淀粉样蛋白生成途径介质(adam10)在mRNA和蛋白质水平上的表达均有所降低。使用苏木精、伊红和 Nissl 染色法进行的组织学评估显示,STZ 给药的鱼体内神经元减少。在 STZ 施用的鱼体内,磷-tauser396/总-tau 的比率增加。基于这些发现,5 毫克/千克体重的 STZ 被认为是表现出 sAD 表型的最合适剂量。质谱分析证实了 STZ 给药鱼的大脑中存在淀粉样 beta 低聚物。透射电子显微镜也显示了高阶不溶性淀粉样蛋白纤维的存在。免疫组化分析显示,STZ 给药鱼的大脑中有淀粉样 beta 沉积。高尔基体-柯克斯染色表明树突数量减少,而小胶质细胞的密度、跨度比、体节面积和裂隙度增加。本研究结果表明,STZ 给药(5 毫克/千克)7 天后,斑马鱼出现了注意力缺失症的特征和表型。这项研究验证了 STZ 诱导的 sAD 在斑马鱼中的潜力,它是研究病理生理学和快速筛选 sAD 治疗分子的可靠模型。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental Neurology
医学-神经科学
CiteScore
10.10
自引率
3.80%
发文量
258
审稿时长
42 days
期刊介绍:
Experimental Neurology, a Journal of Neuroscience Research, publishes original research in neuroscience with a particular emphasis on novel findings in neural development, regeneration, plasticity and transplantation. The journal has focused on research concerning basic mechanisms underlying neurological disorders.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


