Identification of a Non-canonical Function of Prefoldin Subunit 5 in Proteasome Assembly
IF 4.7
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
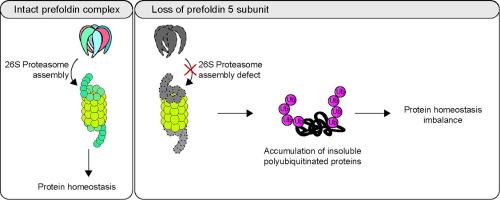
The prefoldin complex is a heterohexameric, evolutionarily conserved co-chaperone that assists in folding of polypeptides downstream of the protein translation machinery. Loss of prefoldin function leads to impaired solubility of cellular proteins. The degradation of proteins by the proteasome is an integral part of protein homeostasis. Failure of regulated protein degradation can lead to the accumulation of misfolded and defective proteins. We show that prefoldin subunit 5 is required for proteasome activity by contributing to the assembly of the 26S proteasome. In particular, we found that absence of the prefoldin subunit 5 impairs formation of the Rpt ring subcomplex of the proteasome. Concomitant deletion of PFD5 and HSM3, a chaperone for assembly of the ATPase subunits comprising the Rpt ring, exacerbates this effect, suggesting a synergistic relationship between the two factors in proteasome assembly. Thus, our findings reveal a regulatory mechanism wherein prefoldin subunit 5 plays a crucial role in maintaining proteasome integrity, thereby influencing the degradation of proteins.
鉴定前折叠素亚基 5 在蛋白酶体组装中的非规范功能。
预折叠素复合物是一种异构六聚体,在进化过程中得到了保守的共伴侣蛋白,可协助蛋白质翻译机制下游的多肽折叠。预折叠素功能的缺失会导致细胞蛋白质的可溶性受损。蛋白酶体对蛋白质的降解是蛋白质平衡不可或缺的一部分。蛋白质降解失调会导致错误折叠和缺陷蛋白质的积累。我们的研究表明,前折叠素亚基 5 是蛋白酶体活性所必需的,它有助于 26S 蛋白酶体的组装。特别是,我们发现预折叠素亚基 5 的缺失会影响蛋白酶体 Rpt 环亚复合物的形成。同时缺失 PFD5 和 HSM3(组成 Rpt 环的 ATPase 亚基的组装伴侣)会加剧这种影响,这表明蛋白酶体组装过程中这两个因子之间存在协同作用关系。因此,我们的研究结果揭示了一种调控机制,即前折叠素亚基 5 在维持蛋白酶体完整性方面发挥着关键作用,从而影响蛋白质的降解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Biology
生物-生化与分子生物学
CiteScore
11.30
自引率
1.80%
发文量
412
审稿时长
28 days
期刊介绍:
Journal of Molecular Biology (JMB) provides high quality, comprehensive and broad coverage in all areas of molecular biology. The journal publishes original scientific research papers that provide mechanistic and functional insights and report a significant advance to the field. The journal encourages the submission of multidisciplinary studies that use complementary experimental and computational approaches to address challenging biological questions.
Research areas include but are not limited to: Biomolecular interactions, signaling networks, systems biology; Cell cycle, cell growth, cell differentiation; Cell death, autophagy; Cell signaling and regulation; Chemical biology; Computational biology, in combination with experimental studies; DNA replication, repair, and recombination; Development, regenerative biology, mechanistic and functional studies of stem cells; Epigenetics, chromatin structure and function; Gene expression; Membrane processes, cell surface proteins and cell-cell interactions; Methodological advances, both experimental and theoretical, including databases; Microbiology, virology, and interactions with the host or environment; Microbiota mechanistic and functional studies; Nuclear organization; Post-translational modifications, proteomics; Processing and function of biologically important macromolecules and complexes; Molecular basis of disease; RNA processing, structure and functions of non-coding RNAs, transcription; Sorting, spatiotemporal organization, trafficking; Structural biology; Synthetic biology; Translation, protein folding, chaperones, protein degradation and quality control.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


