Cryo-EM Structures of the Plasmodium falciparum Apicoplast DNA Polymerase
IF 4.7
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
The apicoplast DNA polymerase (apPol) from Plasmodium falciparum is essential for the parasite’s survival, making it a prime target for antimalarial therapies. Here, we present cryo-electron microscopy structures of the apPol in complex with DNA and incoming nucleotide, offering insights into its molecular mechanisms. Our structural analysis reveals that apPol contains critical residues for high-fidelity DNA synthesis, but lacks certain structural elements to confer processive DNA synthesis during replication, suggesting the presence of additional accessory factors. The enzyme exhibits large-scale conformational changes upon DNA and nucleotide binding, particularly within the fingers and thumb subdomains. These movements reveal potential allosteric sites that could serve as targets for drug design. Our findings provide a foundation for advancing the understanding of apPol’s unique functional mechanisms and potentially offering new avenues for the development of novel inhibitors and therapeutic interventions against malaria.
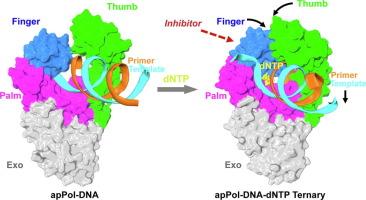
恶性疟原虫 apicoplast DNA 聚合酶的冷冻电镜结构。
恶性疟原虫的 apicoplast DNA 聚合酶(apPol)对寄生虫的生存至关重要,因此成为抗疟疗法的主要靶标。在这里,我们展示了apPol与DNA和传入核苷酸复合物的冷冻电子显微镜结构,为了解其分子机制提供了线索。我们的结构分析表明,apPol含有高保真DNA合成的关键残基,但缺乏某些结构元素,无法在复制过程中进行DNA合成,这表明还存在其他辅助因素。该酶在与 DNA 和核苷酸结合时,尤其是在手指和拇指亚域内,表现出大规模的构象变化。这些变化揭示了潜在的异构位点,可作为药物设计的靶点。我们的发现为进一步了解 apPol 的独特功能机制奠定了基础,并有可能为开发新型抑制剂和疟疾治疗干预措施提供新的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Biology
生物-生化与分子生物学
CiteScore
11.30
自引率
1.80%
发文量
412
审稿时长
28 days
期刊介绍:
Journal of Molecular Biology (JMB) provides high quality, comprehensive and broad coverage in all areas of molecular biology. The journal publishes original scientific research papers that provide mechanistic and functional insights and report a significant advance to the field. The journal encourages the submission of multidisciplinary studies that use complementary experimental and computational approaches to address challenging biological questions.
Research areas include but are not limited to: Biomolecular interactions, signaling networks, systems biology; Cell cycle, cell growth, cell differentiation; Cell death, autophagy; Cell signaling and regulation; Chemical biology; Computational biology, in combination with experimental studies; DNA replication, repair, and recombination; Development, regenerative biology, mechanistic and functional studies of stem cells; Epigenetics, chromatin structure and function; Gene expression; Membrane processes, cell surface proteins and cell-cell interactions; Methodological advances, both experimental and theoretical, including databases; Microbiology, virology, and interactions with the host or environment; Microbiota mechanistic and functional studies; Nuclear organization; Post-translational modifications, proteomics; Processing and function of biologically important macromolecules and complexes; Molecular basis of disease; RNA processing, structure and functions of non-coding RNAs, transcription; Sorting, spatiotemporal organization, trafficking; Structural biology; Synthetic biology; Translation, protein folding, chaperones, protein degradation and quality control.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


