Synthesis, highly potent α-glucosidase inhibition, antioxidant and molecular docking of various novel dihydropyrimidine derivatives to treat diabetes mellitus
IF 2.5
4区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
1,4-dihydropyrimidine-2-thiones were synthesized in five series that include 5-carboxylic acid derivatives of dihydropyrimidine (series A, 6–8), novel 5-carboxamide derivatives of dihydropyrimidine (series B, 9–14), N,S-dimethyl-dihydropyrimidine (series C, 15–20), N-hydrazinyl derivatives of dihydropyrimidine (series D, 21–24) and tetrazolo dihydropyrimidine derivatives (series E, 25–28), and evaluated for anti-diabetic capability. The prepared novel compounds were structurally established by FTIR, 1HNMR, 13CNMR, ESI and HRMS. All of these compounds from series A—E were first time examined for α-glucosidase inhibition as to evaluate their anti-diabetic potential. Most of the compounds for example 8, 11–14, 15, 17–21, 25 and 28 demonstrated greater α-glucosidase inhibitory effects (IC50 = 12.5 ± 0.21 to 47.3 ± 0.23 μM) when compared to deoxynojirimycin as standard (IC50 = 52.02 ± 0.36 μM). Compounds from series B and C found to be highly active however, the compounds from series D found generally less active. The structure–activity relationships demonstrated the importance of C-5 carboxamides, C-5 ethyl ester functionality, and the presence of N,S-dimethyl groups at pyrimidine ring for α-glucosidase inhibition. The docking studies demonstrated that all the active compounds have van der Waals and alkyl bonds interactions with the targeted site of the human lysosomal acid α-glucosidase. All these compounds were also tested for antioxidant potential by DPPH radical scavenging protocol that exhibited significant antioxidant effects (IC50 = 21.4 0.45 to 92.1 0.38 μM) as compared to the standard butylated hydroxyanisol (IC50 = 44.2 0.36 μM). Among all, compound 13, 14 and 19 with potent α-glucosidase inhibition (IC50 = 18.9 ± 0.72, 23.3 ± 0.45 and 21.5 ± 0.16 µM, respectively) along with excellent antioxidant potential in the range of (IC50 = 21.4 0.45 to 31.2 ± 0.23 μM) indicated their ability to use as valuable leads for the development of anti-diabetic drugs with the combined effects of antioxidants.
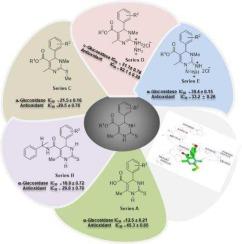
用于治疗糖尿病的各种新型二氢嘧啶衍生物的合成、强效α-葡萄糖苷酶抑制、抗氧化和分子对接。
合成了 1,4-二氢嘧啶-2-硫醚的五个系列,包括二氢嘧啶的 5-羧酸衍生物(系列 A,6-8)、二氢嘧啶的新型 5-甲酰胺衍生物(系列 B,9-14)、二氢嘧啶的 N.S-二甲基衍生物(系列 C,15-20)、二氢嘧啶的 N-肼基衍生物(系列 D,21-24)和二氢嘧啶的四唑、S-二甲基二氢嘧啶(系列 C,15-20)、二氢吡啶的 N-肼基衍生物(系列 D,21-24)和四唑基二氢吡啶衍生物(系列 E,25-28),并对其抗糖尿病能力进行了评估。傅立叶变换红外光谱、1HNMR、13CNMR、ESI 和 HRMS 对制备的新型化合物进行了结构鉴定。对 A-E 系列的所有化合物都进行了首次α-葡萄糖苷酶抑制试验,以评估其抗糖尿病潜力。与标准化合物脱氧野尻霉素(IC50 = 52.02 ± 0.36 μM)相比,大多数化合物(如 8、11-14、15、17-21、25 和 28)显示出更强的α-葡萄糖苷酶抑制作用(IC50 = 12.5 ± 0.21 至 47.3 ± 0.23 μM)。B 系列和 C 系列的化合物活性较高,但 D 系列的化合物活性普遍较低。结构-活性关系表明,C-5 羧酰胺、C-5 乙酯官能团和嘧啶环上的 N,S-二甲基基团对抑制α-葡萄糖苷酶具有重要作用。对接研究表明,所有活性化合物都与人溶酶体酸性α-葡萄糖苷酶的靶位点存在范德华和烷基键相互作用。所有这些化合物还通过 DPPH 自由基清除协议进行了抗氧化潜力测试,与标准丁基化羟基苯甲醚(IC50 = 44.2 ± 0.36 μM)相比,这些化合物具有显著的抗氧化效果(IC50 = 21.4 ± 0.45 至 92.1 ± 0.38 μM)。其中,化合物 13、14 和 19 具有强效的 α-葡萄糖苷酶抑制作用(IC50 分别为 18.9 ± 0.72、23.3 ± 0.45 和 21.5 ± 0.16 µM),同时在(IC50 = 21.4 ± 0.45 至 31.2 ± 0.23 μM)范围内具有出色的抗氧化潜力,这表明它们可以作为开发具有抗氧化剂综合效应的抗糖尿病药物的重要线索。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.70
自引率
3.70%
发文量
463
审稿时长
27 days
期刊介绍:
Bioorganic & Medicinal Chemistry Letters presents preliminary experimental or theoretical research results of outstanding significance and timeliness on all aspects of science at the interface of chemistry and biology and on major advances in drug design and development. The journal publishes articles in the form of communications reporting experimental or theoretical results of special interest, and strives to provide maximum dissemination to a large, international audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


