Structural basis of the C-terminal domain of SARS-CoV-2 N protein in complex with GMP reveals critical residues for RNA interaction
IF 2.2
4区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) nucleocapsid (N) protein performs multiple functions during the viral life cycle, particularly in binding to the viral genomic RNA to form a helical ribonucleoprotein complex. Here, we present that the C-terminal domain of SARS-CoV-2 N protein (N-CTD) specifically interacts with polyguanylic acid (poly(G)). The crystal structure of the N-CTD in complex with 5′-guanylic acid (GMP, also known as guanosine monophosphate) was determined at a resolution of approximately 2.0 Å. A novel GMP-binding pocket in the N-CTD was illustrated. Residues Arg259 and Lys338 were identified to play key roles in binding to GMP through mutational analysis. These two residues are absolutely conserved in the other two highly pathogenic CoVs, SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV). Overall, our findings expand the structural information on N protein interacting with guanylate and reveal a conserved GMP-binding pocket as a potential antiviral target.
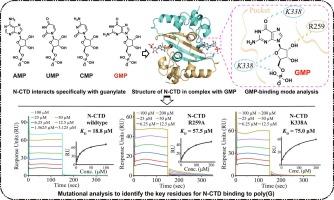
SARS-CoV-2 N 蛋白 C 端结构域与 GMP 复合物的结构基础揭示了 RNA 相互作用的关键残基。
严重急性呼吸系统综合征冠状病毒 2(SARS-CoV-2)核壳(N)蛋白在病毒生命周期中发挥多种功能,特别是与病毒基因组 RNA 结合形成螺旋核糖核蛋白复合物。我们在这里发现,SARS-CoV-2 N 蛋白的 C 端结构域(N-CTD)能与聚鸟苷酸(poly(G))发生特异性相互作用。N-CTD与5'-鸟苷酸(GMP,又称单磷酸鸟苷)复合物的晶体结构分辨率约为2.0埃。结果表明,在 N-CTD 中有一个新的 GMP 结合口袋。通过突变分析,确定 Arg259 和 Lys338 两个残基在与 GMP 结合过程中起着关键作用。这两个残基在另外两种高致病性 CoV--SARS-CoV 和中东呼吸综合征冠状病毒(MERS-CoV)中是绝对保守的。总之,我们的研究结果扩展了 N 蛋白与鸟苷酸相互作用的结构信息,并揭示了一个保守的 GMP 结合口袋是潜在的抗病毒靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.70
自引率
3.70%
发文量
463
审稿时长
27 days
期刊介绍:
Bioorganic & Medicinal Chemistry Letters presents preliminary experimental or theoretical research results of outstanding significance and timeliness on all aspects of science at the interface of chemistry and biology and on major advances in drug design and development. The journal publishes articles in the form of communications reporting experimental or theoretical results of special interest, and strives to provide maximum dissemination to a large, international audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


