Water-soluble neutral octahedral chalcogenide tungsten and molybdenum {M6Q8} clusters with P(C2H4CONH2)3 ligand
IF 3.8
2区 化学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Developing the chemistry of octahedral chalcogenide molybdenum and tungsten cluster complexes in the context of applications in biology and medicine, in this work a series of water-soluble neutral cluster complexes [{M6Q8}(P(C2H4CONH2)3)6] (M = Mo, W; Q = S, Se) have been obtained by simultaneous replacement of inner and terminal halide ligands in [{M6I8}I6]2− with chalcogenide and organic phosphine ligands and characterized by single-crystal X-ray diffraction analysis, 1H and 31P NMR spectroscopies, elemental analysis, and UV–vis spectroscopy. The amide groups of the organic ligands, on the one hand, contribute to the solubility of the resulting clusters in water and, on the other hand, are able to form an extensive network of hydrogen bonds, leading to the crystallization of the complexes from aqueous solutions. Despite this fact, the complexes have sufficient solubility and stability in aqueous solutions, which made it possible to demonstrate their low cytotoxicity on Hep-2 cells (IC50 were not reached even at concentration up to 4 mM). The resulting clusters are among the most biocompatible of the octahedral clusters studied to date and are the starting point for the development of a new family of X-ray contrast agents.
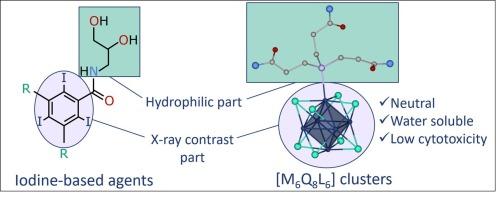
含 P(C2H4CONH2)3 配体的水溶性中性八面体同素钨和钼 {M6Q8} 簇。
在这项工作中,通过同时将[{M6I8}I6]2-中的内配体和末端卤化物配体置换为铬化物和有机膦配体,得到了一系列水溶性中性簇合物[{M6Q8}(P(C2H4CONH2)3)6](M = Mo, W. Q = S, Se;Q = S、Se),并通过单晶 X 射线衍射分析、1H 和 31P NMR 光谱、元素分析和紫外-可见光谱进行表征。有机配体的酰胺基团一方面有助于提高所产生的簇合物在水中的溶解度,另一方面还能形成广泛的氢键网络,从而导致络合物从水溶液中结晶。尽管如此,这些复合物在水溶液中仍有足够的溶解度和稳定性,因此可以证明它们对 Hep-2 细胞的细胞毒性很低(即使浓度达到 4 毫摩尔也达不到 IC50)。由此产生的簇合物是迄今为止所研究的八面体簇合物中生物相容性最好的,也是开发新系列 X 射线造影剂的起点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Inorganic Biochemistry
生物-生化与分子生物学
CiteScore
7.00
自引率
10.30%
发文量
336
审稿时长
41 days
期刊介绍:
The Journal of Inorganic Biochemistry is an established international forum for research in all aspects of Biological Inorganic Chemistry. Original papers of a high scientific level are published in the form of Articles (full length papers), Short Communications, Focused Reviews and Bioinorganic Methods. Topics include: the chemistry, structure and function of metalloenzymes; the interaction of inorganic ions and molecules with proteins and nucleic acids; the synthesis and properties of coordination complexes of biological interest including both structural and functional model systems; the function of metal- containing systems in the regulation of gene expression; the role of metals in medicine; the application of spectroscopic methods to determine the structure of metallobiomolecules; the preparation and characterization of metal-based biomaterials; and related systems. The emphasis of the Journal is on the structure and mechanism of action of metallobiomolecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


