Synthesis of γ-Butyrolactones with Chiral Quaternary-Tertiary Stereocenters via Catalytic Asymmetric Mukaiyama-Michael Addition.
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A catalytic asymmetric Mukaiyama-Michael reaction of silyl ketene acetals (SKAs) with α- or β-substituted α,β-unsaturated pyrazolamides was realized with N,N'-dioxide/nickel(II) complex catalysts. Bidentate coordination of the substrate to the catalyst and elongation of the ligand were beneficial for stereocontrol. In addition, adjustment of the substituents on substrates tuned the reactivity significantly. A wide range of chiral γ-butyrolactones with quaternary-tertiary stereocenters were obtained in moderate to excellent yields, good diastereomeric ratio, and excellent enantiomeric excess values.
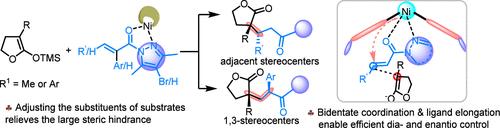
通过催化不对称向山-迈克尔加成法合成具有手性季-叔立体中心的 γ-丁内酯。
在 N,N'-二氧代/镍(II)络合物催化剂的催化下,实现了硅酮乙醛(SKAs)与α-或β-取代的α,β-不饱和吡唑酰胺的不对称向山-迈克尔催化反应。底物与催化剂的双齿配位以及配体的伸长有利于立体控制。此外,调整底物上的取代基也能显著调节反应活性。研究人员以中等到极好的产率、良好的非对映比率和优异的对映体过量值获得了多种具有季-叔立体中心的手性 γ-丁内酯。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


