1,25-Dihydroxyvitamin D3 protects against placental inflammation by suppressing NLRP3-mediated IL-1β production via Nrf2 signaling pathway in preeclampsia
IF 11.9
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
Background
Maternal vitamin D deficiency is associated with an increased risk of preeclampsia, a potentially life-threatening multi-system disorder specific to human pregnancy. Placental trophoblast dysfunction is a key factor in the development of preeclampsia, and the activation of NOD-like receptor protein 3 (NLRP3) inflammasome may play a crucial role in this process. Previous studies have suggested that vitamin D can exert beneficial effects by suppressing inflammasome activation, but the underlying mechanism has not been fully elucidated. This study aims to explore the protective effects of 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] on the placenta and to investigate the mechanisms by which 1,25(OH)2D3 attenuates NLRP3 inflammasome activation in a rat model of preeclampsia and hypoxia-cultured placental trophoblast cells.
Results
Our findings demonstrated that supplementation of rats with 1,25(OH)2D3 mitigated placental inflammation and prevented multi-organ dysfunction associated with preeclampsia. Treatment with 1,25(OH)2D3 inhibited inflammasome-mediated inflammation in trophoblast cells via its receptor VDR by reducing the expression of NLRP3, caspase-1, and apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), decreasing IL-1β production, reducing mitochondrial reactive oxygen species generation, and enhancing the expression and enzymatic activity of Cu/Zn-superoxide dismutase (SOD). Mechanistically, 1,25(OH)2D3 upregulated nuclear factor erythroid 2-related factor 2 (Nrf2) signaling, subsequently suppressing NLRP3-mediated IL-1β overproduction in trophoblast cells.
Conclusions
Our study indicates that 1,25(OH)2D3 inhibits NLRP3-mediated inflammation in trophoblast cells during preeclampsia by stimulating the Nrf2 signaling pathway and inhibiting oxidative stress.
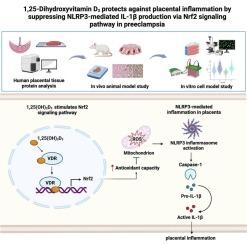
1,25-二羟维生素 D3 通过 Nrf2 信号通路抑制 NLRP3 介导的 IL-1β 生成,从而保护子痫前期患者免受胎盘炎症的影响。
背景:母体维生素D缺乏与子痫前期风险增加有关,子痫前期是人类妊娠期特有的一种可能危及生命的多系统疾病。胎盘滋养细胞功能障碍是子痫前期发病的一个关键因素,而NOD样受体蛋白3(NLRP3)炎性体的激活可能在这一过程中起着至关重要的作用。以往的研究表明,维生素 D 可通过抑制炎性体的激活发挥有益作用,但其潜在机制尚未完全阐明。本研究旨在探讨 1,25-二羟维生素 D3 [1,25(OH)2D3] 对胎盘的保护作用,并研究 1,25(OH)2D3在子痫前期大鼠模型和缺氧培养的胎盘滋养层细胞中抑制 NLRP3 炎性体激活的机制:我们的研究结果表明,给大鼠补充 1,25(OH)2D3可减轻胎盘炎症并预防与子痫前期相关的多器官功能障碍。1,25(OH)2D3通过其受体VDR抑制滋养层细胞中NLRP3、caspase-1和含有caspase募集结构域的凋亡相关斑点样蛋白(ASC)的表达,减少IL-1β的产生,减少线粒体活性氧的生成,提高Cu/Zn-超氧化物歧化酶(SOD)的表达和酶活性,从而抑制滋养层细胞中炎症组介导的炎症。从机理上讲,1,25(OH)2D3能上调核因子红细胞2相关因子2(Nrf2)的信号转导,从而抑制滋养层细胞中NLRP3介导的IL-1β过量产生:我们的研究表明,1,25(OH)2D3可通过刺激Nrf2信号通路和抑制氧化应激,抑制子痫前期滋养层细胞中NLRP3介导的炎症。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Metabolism: clinical and experimental
医学-内分泌学与代谢
CiteScore
18.90
自引率
3.10%
发文量
310
审稿时长
16 days
期刊介绍:
Metabolism upholds research excellence by disseminating high-quality original research, reviews, editorials, and commentaries covering all facets of human metabolism.
Consideration for publication in Metabolism extends to studies in humans, animal, and cellular models, with a particular emphasis on work demonstrating strong translational potential.
The journal addresses a range of topics, including:
- Energy Expenditure and Obesity
- Metabolic Syndrome, Prediabetes, and Diabetes
- Nutrition, Exercise, and the Environment
- Genetics and Genomics, Proteomics, and Metabolomics
- Carbohydrate, Lipid, and Protein Metabolism
- Endocrinology and Hypertension
- Mineral and Bone Metabolism
- Cardiovascular Diseases and Malignancies
- Inflammation in metabolism and immunometabolism
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


