Translation Complex Profile Sequencing Allows Discrimination of Leaky Scanning and Reinitiation in Upstream Open Reading Frame-controlled Translation
IF 4.7
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Upstream open reading frames (uORFs) are a class of translated regions (translons) in mRNA 5′ leaders. uORFs are believed to be pervasive regulators of the translation of mammalian mRNAs. Some uORFs are highly repressive but others have little or no impact on downstream mRNA translation either due to inefficient recognition of their start codon(s) or/and due to efficient reinitiation after uORF translation. While experiments with uORF reporter constructs proved to be instrumental in the investigation of uORF-mediated mechanisms of translation control, they can have serious limitations as manipulations with uORF sequences can yield various artefacts. Here we propose a general approach for using translation complex profiling (TCP-seq) data for exploring uORF regulatory characteristics. Using several examples, we show how TCP-seq could be used to estimate both repressiveness and modes of action of individual uORFs. We demonstrate how this approach could be used to assess the mechanisms of uORF-mediated translation control in the mRNA of several human genes, including EIF5, IFRD1, MDM2, MIEF1, PPP1R15B, TAF7, and UCP2.
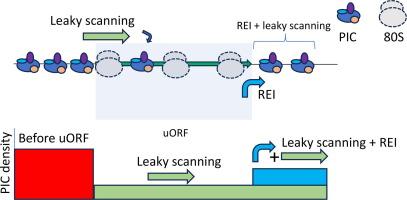
通过翻译复合体轮廓测序,可以分辨上游开放阅读框控制翻译中的漏扫描和再启动。
上游开放阅读框(uORFs)是 mRNA 5' 头部的一类翻译区域(转译子)。据信,uORFs 是哺乳动物 mRNA 翻译的普遍调节因子。有些 uORF 具有高度抑制作用,但有些 uORF 对下游 mRNA 翻译影响很小或没有影响,原因可能是对其起始密码子的识别效率不高,或者/和由于 uORF 翻译后的有效再启动。尽管使用 uORF 报告构建体进行的实验被证明有助于研究 uORF 介导的翻译控制机制,但它们也有严重的局限性,因为对 uORF 序列的操作可能会产生各种假象。在这里,我们提出了一种使用翻译复合剖析(TCP-seq)数据探索 uORF 调控特性的通用方法。通过几个例子,我们展示了如何利用 TCP-seq 估算单个 uORF 的抑制性和作用模式。我们展示了这种方法如何用于评估 uORF 介导的几个人类基因 mRNA 翻译控制机制,包括 EIF5、IFRD1、MDM2、MIEF1、PPP1R15B、TAF7 和 UCP2。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Biology
生物-生化与分子生物学
CiteScore
11.30
自引率
1.80%
发文量
412
审稿时长
28 days
期刊介绍:
Journal of Molecular Biology (JMB) provides high quality, comprehensive and broad coverage in all areas of molecular biology. The journal publishes original scientific research papers that provide mechanistic and functional insights and report a significant advance to the field. The journal encourages the submission of multidisciplinary studies that use complementary experimental and computational approaches to address challenging biological questions.
Research areas include but are not limited to: Biomolecular interactions, signaling networks, systems biology; Cell cycle, cell growth, cell differentiation; Cell death, autophagy; Cell signaling and regulation; Chemical biology; Computational biology, in combination with experimental studies; DNA replication, repair, and recombination; Development, regenerative biology, mechanistic and functional studies of stem cells; Epigenetics, chromatin structure and function; Gene expression; Membrane processes, cell surface proteins and cell-cell interactions; Methodological advances, both experimental and theoretical, including databases; Microbiology, virology, and interactions with the host or environment; Microbiota mechanistic and functional studies; Nuclear organization; Post-translational modifications, proteomics; Processing and function of biologically important macromolecules and complexes; Molecular basis of disease; RNA processing, structure and functions of non-coding RNAs, transcription; Sorting, spatiotemporal organization, trafficking; Structural biology; Synthetic biology; Translation, protein folding, chaperones, protein degradation and quality control.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


