The mode of action of sorafenib in MDA-MB-231 breast carcinoma cells involves components of apoptotic, necroptotic and autophagy-dependent cell death pathways
IF 3.3
3区 生物学
Q3 CELL BIOLOGY
引用次数: 0
Abstract
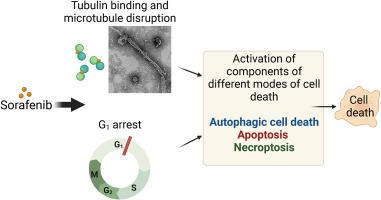
We report the identification of an interesting mode of action by sorafenib (SF) (Nexavar) in triple-negative breast adenocarcinoma MDA-MB-231 cells. The dying cells presented features of apoptosis, such as externalization of phosphatidylserine and cleaved caspase-3, and autophagy-mediated cell death, such as formation of autophagosomes and autolysosomes, the overexpression of LC3-II, and the presence of LAMP1-positive vacuoles, while displaying insufficient autophagic flux. Components of endoplasmic reticulum stress (ER stress; PERK and CHOP) and of necroptosis (p-MLKL) were also elevated considerably. Investigating potential target proteins that could modulate this form of cell death, we next investigated the role of tubulin disruption, which is known to induce necroptosis, apoptosis, and autophagy-dependent cell death. Interactions of SF with purified tubulin were investigated in detail using a combination of cellular and biophysical assays, transmission electron microscopy, and computer simulations. A marked reduction in the intrinsic tryptophan fluorescence of tubulin, a concentration-dependent elevation of anilinonaphthalene sulfonate–tubulin complex fluorescence, electron micrographs of deformed in vitro–assembled microtubules, and disrupted and hyper-stabilized cellular microtubules evinced the ability of SF to target tubulin and disrupt cellular microtubules. Molecular docking and molecular dynamic simulations positioned the drug between the α and β subunits of tubulin with considerable stability (ΔGbind, −31.43 kcal/mol), suggesting that drug-induced perturbation of tubulin could contribute to this mode of cell death.
索拉非尼在 MDA-MB-231 乳腺癌细胞中的作用模式涉及细胞凋亡、坏死和依赖自噬的细胞死亡途径。
我们报告了索拉非尼(SF)(Nexavar)在三阴性乳腺癌 MDA-MB-231 细胞中发现的一种有趣的作用模式。濒死细胞表现出凋亡特征,如磷脂酰丝氨酸外化和裂解的 Caspase-3,以及自噬介导的细胞死亡特征,如自噬体和自溶酶体的形成、LC3-II 的过度表达和 LAMP1 阳性空泡的存在,同时自噬通量不足。内质网应激(ER应激;PERK和CHOP)和坏死(p-MLKL)的成分也显著升高。为了研究可能调控这种细胞死亡形式的潜在靶蛋白,我们接下来研究了微管蛋白破坏的作用,众所周知,微管蛋白破坏会诱导坏死、细胞凋亡和依赖自噬的细胞死亡。我们结合细胞和生物物理实验、透射电子显微镜和计算机模拟,详细研究了 SF 与纯化的微管蛋白之间的相互作用。小管蛋白固有色氨酸荧光的显著降低、萘磺酸苯胺-小管蛋白复合物荧光浓度依赖性的升高、体外组装微管变形的电子显微镜照片以及破坏和超稳定的细胞微管都证明了 SF 靶向小管蛋白和破坏细胞微管的能力,其对细胞存活率的影响为 IC50。分子对接和分子动力学模拟将这种药物定位在微管蛋白的 α 和 β 亚基之间,具有相当高的稳定性(ΔGbind,-31.43 kcal/mol),表明药物诱导的微管蛋白扰动可能有助于这种细胞死亡模式。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental cell research
医学-细胞生物学
CiteScore
7.20
自引率
0.00%
发文量
295
审稿时长
30 days
期刊介绍:
Our scope includes but is not limited to areas such as: Chromosome biology; Chromatin and epigenetics; DNA repair; Gene regulation; Nuclear import-export; RNA processing; Non-coding RNAs; Organelle biology; The cytoskeleton; Intracellular trafficking; Cell-cell and cell-matrix interactions; Cell motility and migration; Cell proliferation; Cellular differentiation; Signal transduction; Programmed cell death.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


