7-Hydroxycoumarin and its conjugated metabolites interact with organic anion transporters 1 and 3 in vitro and in vivo
IF 4.7
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
7-Hydroxycoumarin (7-HC) is a natural coumarin compound rich in Chinese herbal medicines and has various pharmacological activities. After oral administration of 7-HC in rodents, its conjugated metabolites 7-hydroxycoumarin-β-D-glucuronide (7-HCG) and 7-hydroxycoumarin sulfate (7-HCS), exhibit high systemic exposure and urinary excretion. Organic anion transporters 1 and 3 (OAT1 and OAT3), mainly expressed in the proximal renal tubules, play an important role in drug-drug interactions and drug-induced kidney injury. We aimed to explore the mechanisms of OAT-mediated drug interactions and renal protective mechanisms of 7-HC and its conjugates. OAT-overexpressing cell models revealed that 7-HC was not a substrate for OAT1 and OAT3, while 7-HCG was specifically transported by OAT3. In contrast, 7-HCS can be transported by both OATs. Besides, 7-HC significantly inhibited the activity of OAT1 and OAT3, while 7-HCS had a strong inhibitory effect on OAT1 (IC50 < 10 μM). After co-administration of 100 mg/kg of 7-HC to mice, systemic exposure and clearance of furosemide (a clinical substrate of OATs) were significantly increased and decreased, respectively. In addition, 7-HC decreased OAT-mediated cytotoxicity and reduced the renal distribution of adefovir in mice. Together, these findings will provide support for OAT-mediated drug interactions and the renal protection of 7-HC.
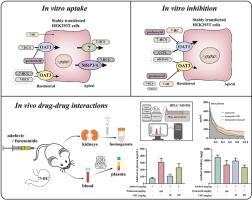
7-羟基香豆素及其共轭代谢物在体外和体内与有机阴离子转运体 1 和 3 相互作用。
7-羟基香豆素(7-HC)是一种富含于中药材中的天然香豆素化合物,具有多种药理活性。啮齿动物口服 7-HC 后,其共轭代谢物 7- 羟基香豆素-β-D-葡萄糖醛酸苷(7-HCG)和 7- 羟基香豆素硫酸盐(7-HCS)表现出较高的全身暴露量和尿排泄量。有机阴离子转运体 1 和 3(OAT1 和 OAT3)主要在近端肾小管中表达,在药物间相互作用和药物引起的肾损伤中发挥着重要作用。我们旨在探索 OAT 介导的药物相互作用机制以及 7-HC 及其共轭物的肾脏保护机制。OAT过表达细胞模型显示,7-HC不是OAT1和OAT3的底物,而7-HCG则由OAT3特异性转运。相反,7-HCS 可被两种 OAT 转运。此外,7-HC 能明显抑制 OAT1 和 OAT3 的活性,而 7-HCS 对 OAT1 有很强的抑制作用(IC50
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.70
自引率
3.90%
发文量
410
审稿时长
36 days
期刊介绍:
Chemico-Biological Interactions publishes research reports and review articles that examine the molecular, cellular, and/or biochemical basis of toxicologically relevant outcomes. Special emphasis is placed on toxicological mechanisms associated with interactions between chemicals and biological systems. Outcomes may include all traditional endpoints caused by synthetic or naturally occurring chemicals, both in vivo and in vitro. Endpoints of interest include, but are not limited to carcinogenesis, mutagenesis, respiratory toxicology, neurotoxicology, reproductive and developmental toxicology, and immunotoxicology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


